Abstract
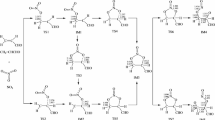
THE inhibiting effect of nitric oxide upon radical chain-reactions in the decomposition of certain organic compounds is well known1, and such mechanisms as CH3 + NO → CH3NO → H2CNOH → HCN + H2O have been invoked to explain this inhibition. Although Küchler and Theile2 obtained traces of cyanide from the nitric oxide-inhibited decomposition of ethane, in no case has the nitroso compound been isolated in such reactions. Coe and Doumani3 prepared nitrosomethane from the photolysis of tert.-butyl nitrite.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Hinshelwood, “The Kinetics of Chemical Change”, 92 (Oxford, 1940).
Küchler and Theile, Z. phys. Chem., B42, 359 (1939).
Coe and Doumani, J. Amer. Chem. Soc., 70, 1516 (1948).
Feigl, “Spot Tests”, 267 and 290 (Amsterdam, 1939).
Braude, Ann. Rep. Chem. Soc., 42, 112 (1945).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
CHILTON, H., GOWENLOCK, B. Reaction of Nitric Oxide with Gaseous Hydrocarbon Free Radicals. Nature 172, 73 (1953). https://doi.org/10.1038/172073a0
Issue date:
DOI: https://doi.org/10.1038/172073a0