Abstract
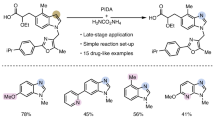
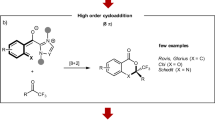
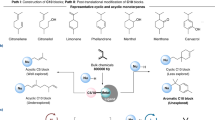
IT has been observed that upon attempted distillation of the oxime of 2-phenylcyclohept-2-enone in a high vacuum, thermal cyclization takes place with the formation of cycloheptenoindole1. If the generality of this reaction could be demonstrated, a valuable new route would be available towards obtaining certain natural products containing the indole nucleus, employing starting materials more readily available than those containing a preformed indole system. We wish to report the extension of the thermal cyclization reaction of oximes to a number of new cases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Ginsburg, D., and Pappo, R., J. Amer. Chem. Soc., 75, 1094 (1953).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
LÖFFLER, A., GINSBURG, D. A New Synthesis of Carbazoles. Nature 172, 820 (1953). https://doi.org/10.1038/172820b0
Issue date:
DOI: https://doi.org/10.1038/172820b0