Abstract
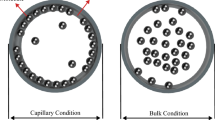
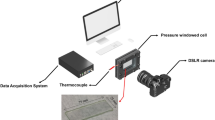
A CONSIDERABLE amount of experimental work on freezing-point depressions of capillary condensates has been reported in the literature in recent years1. Carman2 has given an account of a few more properties in which capillary-held liquids differ from the same materials in bulk conditions. Boiling points of condensates, however, do not appear to have been studied in detail. We have devised a simple method for this purpose. The apparatus, in its essentials, consists of a small vessel, which contains the capillary system under examination; it is connected to a vacuum pump as well as to a suitable differential manometer and is placed in an oil-bath, the temperature of which can be gradually raised. The vessel is first evacuated, and the manometer then registers the vapour pressure of the liquid under examination. The temperature of the bath is then gradually raised until the vapour pressure becomes equal to the atmospheric pressure. The temperature recorded then is the required boiling point.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Batchelor, R. W., and Foster, A. G., Trans. Farad. Soc., 40, 300 (1944). Milligan, W. O., and Rachford, H., J. Amer. Chem. Soc., 70, 2922 (1948). Brown, M. J., and Foster, A. G., Nature, 169, 37 (1952). Iwakami, Y., J. Chem. Soc. (Japan), Pure Chem. Section, 72, 707 (1951). Higuiti, I., and Shimizu, M., J. Phys. Chem., 56, 198 (1952).
Carman, C. P., J. Phys. Chem., 57, 56 (1953).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
LAKHANPAL, M., PURI, B. Boiling Point of Capillary-condensed Water. Nature 172, 917 (1953). https://doi.org/10.1038/172917a0
Issue date:
DOI: https://doi.org/10.1038/172917a0