Abstract
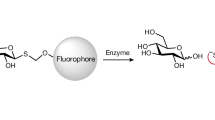
IN the course of a study of the mechanisms involved in the solvolytic reactions of glycosides, we have investigated the position of bond fission occurring in the hydrolysis of α- and β-D-methylglucosides. The reactions were carried out in water enriched in oxygen-18, and the methanol produced was isolated by fractional distillation in an efficient column, and then pyrolysed to give carbon monoxide which was examined by a mass spectrometer. We have found that the hydrolysis of α- and β-D-methylglucosides both in 1 N acid solution and in the presence of appropriate methylglucosidases proceeds by fission of the hexose–oxygen bond: 
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Armstrong, J. Chem. Soc., 1305 (1903).
Koshland, Biol. Rev., 416 (1953).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
BUNTON, C., LEWIS, T., LLEWELLYN, D. et al. Hydrolysis of Methylglucosides. Nature 174, 560 (1954). https://doi.org/10.1038/174560a0
Issue date:
DOI: https://doi.org/10.1038/174560a0
This article is cited by
-
Substrate Cleavage Point with Glucamylase
Nature (1962)