Abstract
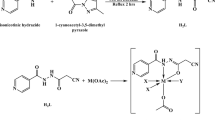
IN our earlier report we directed attention to the importance of chelation as an essential step in the anti-tuberculous activity of isonicotinic hydrazide (isoniazid)1. It was impossible to decide whether the chelating action with a metal or metals took place before (in the medium) or after adsorption on the bacterial cell.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Cymerman-Craig, Rubbo, Willis and Edgar, Nature, 176, 34 (1955).
Rubbo, Albert and Gibson, Brit. J. Exp. Path., 31, 425 (1950).
Rubbo and Pierson, Amer. Rev. Tuberc., 68, 48 (1953).
Rubbo and Cymerman-Craig, Nature, 176, 887 (1955).
Cymerman-Craig, Rubbo and Pierson, Brit. J. Exp. Path., 35, 478 (1954).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
RUBBO, S., EDGAR, J., CYMERMAN-CRAIG, J. et al. Mode of Action of Isonicotinic Hydrazide. Nature 177, 480 (1956). https://doi.org/10.1038/177480a0
Issue date:
DOI: https://doi.org/10.1038/177480a0
This article is cited by
-
Biochemische Reaktionsmöglichkeiten des Isoniazids
Beiträge zur Klinik der Tuberkulose und spezifischen Tuberkulose-Forschung (1957)