Abstract
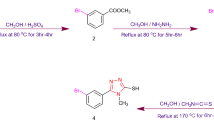
IN the course of investigations on the synthesis of 2-amino-4-aryl thiazoles and their derivatives, it was decided to examine their fungicidal properties, as they contain a cyclic grouping, —N—C—S—, which is present in the structure of some of the well-known fungicides1, and the presence of lipophilic and polar substituents like aryl- and amino-groups in the thiazole nucleus is expected to increase the fungi-toxic properties of the thiazoles as reported earlier2. The recent use of some thiazole derivatives as fungicides3 has greatly stimulated work in this direction. The introduction of halogens into the benzene and other aromatic nuclei of various phenolic compounds greatly increases their fungicidal properties4, and as bromo-derivatives are often more potent than the chloro-compounds, it was thought worth while to introduce bromine into the thiazole nuclei with the hope of augmenting their fungicidal and other activities, in the present communication, seventeen different 2-amino-4-aryl (phenyl ; p-tolyl ; p-ethyl phenyl ; p-methoxy and p-ethoxy phenyl ; β-phenylethyl and β((p-methoxy)phenyl)ethyl ; 2 : 5 dimethyl phenyl and 3 : 4 dimethylphenyl ; α- and β-naphthyl ; o- and p-hydroxy phenyl ; m- and p-amino phenyl ; p-bromo phenyl and 2-thienyl) thiazoles have been prepared by the method of King and Hlavacek5 by allowing different aryl methyl ketones to react with thiourea in the presence of iodine. These compounds have been brominated with bromine in acetic acid medium after protecting the amino-group either by converting it to the hydro-bromide salt or by acetylating it. In all these cases bromine entered position 5 of the thiazole nucleus. The position of bromine was ascertained by the inability of such compounds to undergo coupling with diazotized aniline, which usually takes place at position 56 if it is free.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Marsh, R. W., Ann. App. Biol., 25, 583 (1938). Tisdale, W. H., and Williams, I., U.S. Patent, 1,972,961 (1934). Steiger, N., and Keller, O., U.S. Pat. 2,578,757, Dec. 18 (1951).
Horsfall, J. G., and Rich, S., Contrib. Boyce Thompson Inst., 16, 313 (1951).
Schmitt, C. G., Contrib. Boyce Thompson Inst., 16, 261 (1951).
Byrde, R. J. W., and Woodcock, D., Nature, 169, 503 (1952).
King, L. C., and Hlavacek, R. J., J. Amer. Chem. Soc., 72, 3722 (1950).
Beyer, H., and Wolter, G., Chem. Ber., 85, 1077 (1952).
Montgomery, H. B. S., and Moore, M. H., J. Pom. and Hort. Sci., 15, 253 (1938).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
MAHAPATRA, G. Non-metallic Fungicides. Nature 177, 938 (1956). https://doi.org/10.1038/177938a0
Issue date:
DOI: https://doi.org/10.1038/177938a0