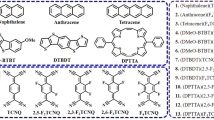
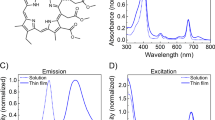
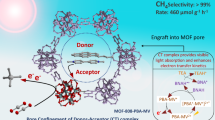
Abstract
IT has been shown by several workers1–4 that there is a rough correlation between the lowest ionization potential of an electron donor and the frequency of the maximum of the charge-transfer band (νCT) of lowest energy characteristic of the complex of the donor with a given acceptor. That the relation is not a very strict one is illustrated by Fig. 1, where such a plot is given for chloranil complexes in solution in carbon tetrachloride. The ionization potentials are those measured by Watanabe5 using a photo-ionization method.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
McConnell, H., Ham, J. S., and Platt, J. R., J. Chem. Phys., 21, 66 (1058).
Keefer, R. M., and Andrews, L. J., J. Amer. Chem. Soc., 74, 4500 (1952).
Briegleb, G., and Czekalla, J., Z. Electrochem., 59, 184 (1955).
Bier, A., Rec. Trav. Chim., 75, 866 (1956).
Watanabe, K., J. Phys. Chem., 22, 1564 (1954); 26, 542 (1957).
de Maine, P. A. D., J. Phys. Chem., 26, 1189 (1957).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
FOSTER, R. Absorption Spectra of Molecular Complexes. Nature 181, 337–338 (1958). https://doi.org/10.1038/181337a0
Issue date:
DOI: https://doi.org/10.1038/181337a0
This article is cited by
-
Molecular complexes of halogens and interhalogen compounds with oxygen-containing compounds
Theoretical and Experimental Chemistry (1981)
-
Concept of ?-surplus character in the chemistry of heteroaromatic compounds (review)
Chemistry of Heterocyclic Compounds (1977)
-
Heterocyclic analogs of pleiadiene
Chemistry of Heterocyclic Compounds (1976)
-
Heterocyclic pleiadiene analogs
Chemistry of Heterocyclic Compounds (1971)
-
Absorption spectra of some molecular complexes
The Science of Nature (1962)