Abstract
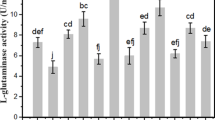
THE glutamic–aspartic transaminase of pig heart muscle has been more than 98 per cent resolved into apoenzyme and co-factor by carrying out a differential heat inactivation in phosphate buffer in the course of purification of the enzyme. The apoenzyme has been purified 64-fold, by relatively simple and reproducible techniques. The highest purification factor previously reported1 for the apoenzyme is 26. The specific activity of the purified apoenzyme compares favourably with that of an electrophoretically homogeneous preparation of the holoenzyme2. Cammarata and Cohen's suggestion3 that purification of the apoenzyme necessarily results in partial inactivation has been disproved, since it is possible to account for all the enzyme activity among the various protein fractions obtained at each step in the purification.
Similar content being viewed by others
Article PDF
References
O'Kane and Gunsalus, J. Biol. Chem., 170, 425 (1947).
Lis, Biochim. Biophys. Acta, 28, 191 (1958).
Cammarata and Cohen, J. Biol. Chem., 193, 53 (1951).
Cohen, J. Biol. Chem., 136, 565, 585 (1940).
Bonavita and Scardi, Experientia, 14, 7 (1958).
Metzler, Ikawa and Snell, J. Amer. Chem. Soc., 76, 648 (1954).
Meister, Sober and Peterson, J. Biol. Chem., 206, 89 (1954).
Braunstein, “Adv. in Protein Chem.”, 3, 1 (1947).
Krebs, Biochem. J., 54, 82 (1953).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
BANKS, B., OLDHAM, K., THAIN, E. et al. Glutamic–Aspartic Transaminase of Pig Heart Muscle. Nature 183, 1187 (1959). https://doi.org/10.1038/1831187a0
Issue date:
DOI: https://doi.org/10.1038/1831187a0