Abstract
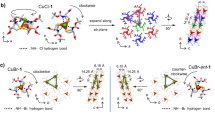
IN previous communications from this laboratory1–3 evidence has been presented that acid dissociations occur from the 1 : 1 cupric complexes of histidine and histamine. In the presence of equimolar cupric chloride, one equivalent of alkali additional to that needed by either of the ligands alone is required to titrate all the acid produced. When the ligand/ copper(II) ratio is 2 : 1 the amount of alkali required is the same as in the absence of the metal. From these facts it was concluded2 that a co-ordinated water molecule and not the hydrogen attached to the uncoordinated imidazole nitrogen was the source of the acid ionization from the 1 : 1 complexes. As a further test of this idea, we have investigated the copper(II) complexes of 3-methyl histamine.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Datta, S. P., Leberman, R., and Rabin, B. R., Biochem. J., 68, 22P (1958).
Leberman, R., and Rabin, B. R., Nature, 183, 746 (1959).
Leberman, R., and Rabin, B. R., Trans. Farad. Soc., 55, 1660 (1959).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
LEBERMAN, R., RABIN, B. Copper(II) Complexes of Histamine and 3-Methyl Histamine. Nature 185, 768 (1960). https://doi.org/10.1038/185768a0
Issue date:
DOI: https://doi.org/10.1038/185768a0
This article is cited by
-
Naming of substituted histamines
Experientia (1974)