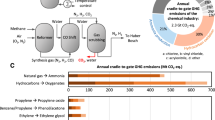
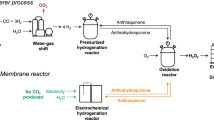
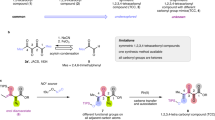
Abstract
CURRENT schemes for explaining chain-growth in the Fischer–Tropsch synthesis1–4 do not permit the formation of an ethyl-branched hydrocarbon. We wish to report the identification of 3-ethylpentane in a hydrogenated Fischer–Tropsch synthesis product. A compound with this carbon skeleton has not hitherto been found in the product. Quantitative analyses by gas chromatography give a mole ratio of 2,3-dimethylpentane to 3-ethylpentane of 1.5. Weitkamp1 found that 2,3-dimethylpentane constituted 0.9 volume per cent of the hydrogenated C7-hydrocarbons from the synthesis product.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Weitkamp, A. W., Seelig, H. S., Bowman, N. J., and Cady, W. E., Indust. Eng. Chem., 45, 343 (1953). Weitkamp, A. W., and Frye, C. G., ibid., 45, 363 (1953).
Anderson, R. B., “Catalysis”, edit. by Emmett, P. H., 4, 257 (Reinhold, New York, 1956).
Wender, I., Friedman, S., Steiner, W. A., and Anderson, R. B., Chem. and Indust., 1694 (1958).
Gall, D., and Kipping, P. J., J. Inst. Petrol., 44, 243 (1958).
Hall, W. K., Kokes, R. J., and Emmett, P. H., J. Amer. Chem. Soc., 82, 1027 (1960).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
BLAUSTEIN, B., WENDER, I. & ANDERSON, R. Ethyl Branching in the Fischer–Tropsch Synthesis. Nature 189, 224–225 (1961). https://doi.org/10.1038/189224a0
Issue date:
DOI: https://doi.org/10.1038/189224a0