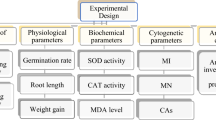
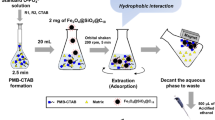
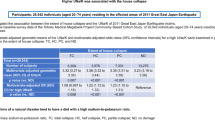
Abstract
A RADIOCHEMICAL method1 has been developed for the determination of cæsium-137 in urine. Acidified urine containing cæsium carrier is passed through a column of ammonium phosphomolybdate mixed with an equal weight of asbestos to give adequate porosity. The ammonium phosphomolybdate acts as a highly selective cation-exchanger and retains the cæsium quantitatively, even at comparatively high flow-rates. Other cations, including sodium, potassium and the alkaline earths, are not retained to any significant extent. After all the sample has passed through the column, the ammonium phosphomolybdate is dissolved in sodium hydroxide solution and the cæsium precipitated as the cobaltinitrite. The removal of final traces of potassium and rubidium from the cæsium is ensured by its subsequent precipitation as the bismuth iodide complex and finally as the chloroplatinate, which is filtered, dried and beta-rays counted. The recovery of carrier normally exceeds 80 per cent. The sensitivity of this method makes it particularly useful for the determination of fall-out cæsium-137 in urine, as current concentrations are low and are likely to decline still further.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Arkell, G. M., and Morgan, A., Atom. Energy Res. Est., Harwell, R. 3675 (1961).
Miller, C. E., and Marinelli, L. D., Argonne Nat. Lab. Rep. ANL-5919, 74 (1958).
McNeill, K. G., and Trojan, O. A. D., Health Phys., 4, 109 (1960).
Schulert, A. R., Nature, 189, 933 (1961).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
MORGAN, A., ARKELL, G. Determination of Cæsium-137/Potassium Ratios in Diet and in the Human Body by Urine Analysis. Nature 191, 1100 (1961). https://doi.org/10.1038/1911100a0
Issue date:
DOI: https://doi.org/10.1038/1911100a0