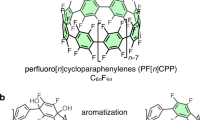
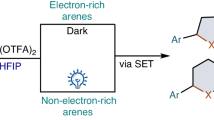
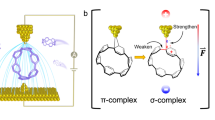
Abstract
THE peaks in the mass spectra of aliphatic saturated fluorocarbons corresponding to the molecule-ions have been described as vanishingly small1. Under normal conditions of electron bombardment, less than 0.1 per cent of the total ion current is due to the molecule-ion so that determinations of the ionization potentials of these compounds by the electron impact method is not possible. It was shown recently in a mass spectrometric examination of cyclic fluorocarbons2 that the molecule-ions of the perfluorocycloalkanes were at least ten times as abundant as those of the corresponding perfluoroalkanes, and it has thus been found possible by working at high pressures in the ion source (1 × 10−5 mm.) to determine the ionization potentials of perfluorocyclopentane and perfluorocyclohexane. The precision of the determination of the ionization potential of an ion of low abundance is less than that which is normally claimed for the electron impact method and is probably of the order of ±0.3 eV.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Mohler, F. L., Dibeler, V. H., and Reese, R. M., J. Res. Nat. Bur. Stand., 49, 343 (1952).
Majer, J. R., J. App. Chem., 11, 141 (1961).
Majer, J. R., Patrick, C. R., and Robb, J. C., Trans. Farad. Soc., 57, 14 (1961).
Majer, J. R., Advances in Fluorine Chemistry, 2 (Butterworths, London, 1961).
Goldstein, L., Ann. Phys., 9, 723 (1938).
Stokes, S., and Duncan, A. B. T., J. Amer. Chem. Soc., 80, 6177 (1958).
Hildebrand, J. L., and Scott, R. L., Solubility of Nonelectrolytes (Reinhold, New York, 1950).
Scott, R. L., J. Phys. Chem., 62, 136 (1958).
Reed, T. M., J. Phys. Chem., 59, 425 (1955).
Munn, R. J., Trans. Farad. Soc., 57, 187 (1961).
Hudson, G. H., and McCoubrey, J. C., Trans. Farad. Soc., 56, 761 (1960).
Nat. Bur. Stand., Circ. 467., 3 (1958).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
MAJER, J., PATRICK, C. Ionization Potentials of Perfluorocycloalkanes. Nature 193, 161–162 (1962). https://doi.org/10.1038/193161b0
Issue date:
DOI: https://doi.org/10.1038/193161b0