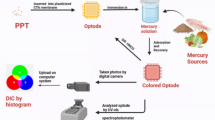
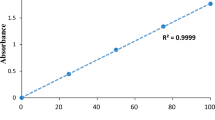
Abstract
THE well-documented hypsochromic and bathochromic shifts observed in the wave-length maxima of the transmittance spectra of many solutes can be explained in many cases on the basis of solute–solvent interactions in which the polarity of the solvent and/or solute is an important factor in determining the type and magnitude of the shift1,2.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
McRae, E. H., J. Phys. Chem., 61, 562 (1957).
Brooker, L. S., and Sprague, R. H., J. Amer. Chem. Soc., 63, 3214 (1941).
Zeitlin, H., and Goya, H., Nature, 183, 1041 (1959).
Goya, H., Waugh, J. L. T., and Zeitlin, H., J. Phys. Chem., 66, 1206 (1962).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
ZEITLIN, H., GOYA, H. & WAUGH, J. Reflectance Spectra of Some Mercury (II) Compounds on Active Adsorbents. Nature 198, 178–179 (1963). https://doi.org/10.1038/198178b0
Issue date:
DOI: https://doi.org/10.1038/198178b0