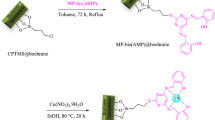
Abstract
IT has been established that copper(II) will adopt an approximately tetrahedral environment under certain circumstances but evidence on the exact geometry of this configuration is still meagre. That which is available, with two possible exceptions1,2, shows a stereochemistry which is more nearly planar than tetrahedral3. For instance, in 2,2′-biphenylbis-(2-iminomethylenephenolato) copper(II), where copper is complexed by a tetradentate molecule with donor atoms which are naturally tetrahedrally disposed, the ligand is considerably distorted and the expected 90° angle between the two planes containing the salicylaldimine groups is reduced to 43°.
This is a preview of subscription content, access via your institution
Access options
Similar content being viewed by others
References
Sacconi, L., and Ciampolini, M., J. Chem Soc., 276 (1964).
Brown, D. H., and Mair, J. A., J. Chem Soc., 3946 (1962).
Cheeseman, T. P., Hall, D., and Waters, T. N., Proc. Chem. Soc., 379 (1963).
Fox, M. R., Lingafelter, E. C., Onioli, P. L., and Sacconi, L., Nature, 197, 1104 (1963).
Sim, G. A., Acta Cryst., 12, 813 (1959); 13, 511 (1960).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
CHEESEMAN, T., HALL, D. & WATERS, T. Stereochemistry of Copper in Bis(N-t-butylsalicylaldiminato)copper(II). Nature 205, 494–495 (1965). https://doi.org/10.1038/205494b0
Published:
Issue date:
DOI: https://doi.org/10.1038/205494b0
This article is cited by
-
Ligand-to-Metal Charge Transfer Resulting in Metalloaromaticity of [R,R-Cyclohexyl-1,2-bis(2-Oxidonaphthylideneiminato-N,N′,O,O′)]Cu(II): A Scrutinized Structural Investigation
Journal of Inorganic and Organometallic Polymers and Materials (2010)
-
Co(II) and Cu(II) Schiff base complexes of bis(N-(4-diethylamino-2-methylphenyl)-3,5-di-tert-butylsalicylaldimine): Electrochemical and X-ray structural study
Structural Chemistry (2008)
-
Stereochemistry of the copper atom in salicylaldiminate complexes
Journal of Structural Chemistry (1978)