Abstract
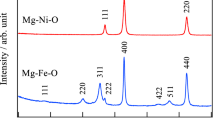
HIGH-PURITY (99.999 per cent) magnesium oxide was prepared by the thermal decomposition of pure magnesium oxalate dihydrate. Details of the preparation, analyses and thermal decomposition of the magnesium oxalate have been given elsewhere1,2. Prior to the X-ray exam ination, the magnesium oxide was recrystallized by firing as a pressed disk at 1,500° C for 24 h. X-ray powder diffraction data were obtained by means of a 19 cm diameter camera using copper Kα-radiation with a nickel filter, and standard procedures. The observed spacings are given in Table 1.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Brown, R. A., thesis, Univ. London (1963).
Brown, R. A., Amer. Ceram. Soc., Bull., 44 (6), 483 (1965).
Nelson, J. B., and Riley, D. P., Proc. Phys. Soc., 57, 160 (1945).
Swanson, H. E., and Tatge, E., JC Fel. Reports, NBS (1949).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
BROWN, R. X-ray Studies on Highly Pure Magnesium Oxide. Nature 208, 481 (1965). https://doi.org/10.1038/208481a0
Issue date:
DOI: https://doi.org/10.1038/208481a0
This article is cited by
-
The Mg−O (Magnesium-Oxygen) system
Bulletin of Alloy Phase Diagrams (1987)