Abstract
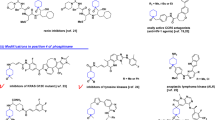
IN the course of our work on this class of compound an animal-anthelmintic1 and a selective herbicide2 had previously been discovered. Common to the structures of the two compounds was the linking of the phosphor-amidothionate group (I) to a chlorinated phenyl ring by an oxygen atom. Our speculations on biological activity led us to replace this atom by nitrogen and to investigate the effects of a different type of toxophoric phosphor-amidothionate group (II). 
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Landram, J. F., and Shaver, R. J., J. Parasitol., 45, 55 (1959).
Leasure, K., U.S. Patent 3,074,790 (1963).
Tolkmith, H., U.S. Patents 2,964,524 (1960) and 2,967,180 (1961).
Tolkmith, H., J. Amer. Chem. Soc., 84, 2097 (1962).
Tolkmith, H., Ann. N.Y. Acad. Sci., 136, 59 (1966).
Dauterman, W. C., Casida, J. E., Knaak, J. B., and Kowalczyk, T., J. Agric. Food Chem., 7, 188 (1959).
Tolkmith, H., and Senkbeil, H. O., Bleg. Patent 661,891 (1965).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
TOLKMITH, H. Biological Activity of Phosphoramidothionates. Nature 211, 522–523 (1966). https://doi.org/10.1038/211522a0
Issue date:
DOI: https://doi.org/10.1038/211522a0
This article is cited by
-
The development of synthetic fungicides; trends and prospects
Netherlands Journal of Plant Pathology (1969)