Abstract
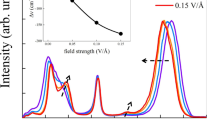
THE formation of hydrated electrons involves electronic and orientation polarizations. This is quantitatively described by  where ΔE is the transfer energy, A is a coefficient, εop and εs are the optical and the static dielectric constants, respectively. The orientation polarization, which mainly determines the value of εs in polar liquids, changes as the reciprocal of the temperature. As the temperature decreases, therefore, the maximum of the absorption band in liquid water shifts to the short-wave region2. The formation of a rigid lattice at the point of the phase transition from liquid to ice sharply decreases the mobility of the dipoles. Values measured for fast processes εs in equation 1 should be substituted by the values measured in an a.c. field of suitable frequency; for processes involving hydrated electrons, τ ∼ 10−6–10−4 sec. At − 10° C, ε is between 3 and 20 if measured at a suitable frequency (106–104 c/s) (ref. 3). Equation 1 then gives ΔE equal to between 1.45 and 0.45 eV, which correspends to a λmax between 9,000 and 40,000 Å. If this reasoning were correct, the absorption maximum would be expected to shift into the infra-red when the temperature is below 0° C.
where ΔE is the transfer energy, A is a coefficient, εop and εs are the optical and the static dielectric constants, respectively. The orientation polarization, which mainly determines the value of εs in polar liquids, changes as the reciprocal of the temperature. As the temperature decreases, therefore, the maximum of the absorption band in liquid water shifts to the short-wave region2. The formation of a rigid lattice at the point of the phase transition from liquid to ice sharply decreases the mobility of the dipoles. Values measured for fast processes εs in equation 1 should be substituted by the values measured in an a.c. field of suitable frequency; for processes involving hydrated electrons, τ ∼ 10−6–10−4 sec. At − 10° C, ε is between 3 and 20 if measured at a suitable frequency (106–104 c/s) (ref. 3). Equation 1 then gives ΔE equal to between 1.45 and 0.45 eV, which correspends to a λmax between 9,000 and 40,000 Å. If this reasoning were correct, the absorption maximum would be expected to shift into the infra-red when the temperature is below 0° C.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Pekar, S., and Eksperim, Zh., I Teor. Fiz., 17, 868 (1947).
Baxendale, J. H., Zh. Mendeleev Soc., 11, 168 (1966).
Landolt, 6, 453 (1959).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
SHUBIN, V., ZHIGUNOV, V., ZOLOTAREVSKY, V. et al. Pulse Radiolysis of Crystalline Ice and Frozen Crystalline Aqueous Solutions. Nature 212, 1002 (1966). https://doi.org/10.1038/2121002a0
Received:
Issue date:
DOI: https://doi.org/10.1038/2121002a0
This article is cited by
-
Trapped electrons in crystalline media
Research on Chemical Intermediates (1989)
-
Electrochemical Detection of Excited States during Pulse Radiolysis of Crystalline Ice
Nature Physical Science (1971)
-
Kinetics of thermal and photostimulated destruction of captured electrons in?-irradiated glassy alkaline ice
Bulletin of the Academy of Sciences of the USSR Division of Chemical Science (1970)
-
Optical absorption of neutral crystalline ice, ?-irradiated at ?196�
Bulletin of the Academy of Sciences of the USSR Division of Chemical Science (1968)
-
Solvated Electron Spectrum in Irradiated Ice
Nature (1967)