Abstract
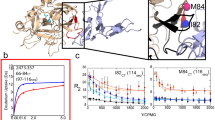
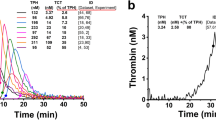
THE original work on the activity of thrombin on synthetic substrates1 indicated that its hydrolytic action was confined to the arginine esters and amides, but more recent investigations2 have shown that the specificity of thrombin is very similar to that of trypsin, which hydrolyses both arginine and lysine esters. We have recently reported that thrombin also hydrolyses phenylalanine and tyrosine ester substrates (unpublished results), and we have therefore investigated the enzyme activity of thrombin towards a variety of amino-acid esters, to define more precisely the specificity of the enzyme.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Sherry, S., and Troll, W., J. Biol. Chem., 208, 95 (1954).
Elmore, D. T., and Curragh, E. F., Biochem. J., 93, 163 (1964).
Seegers, W. H., Cole, E. R., Harmison, C. R., and Marciniak, E., Canad. J. Biochem. Physiol., 41, 1047 (1963).
Seegers, W. H., and Smith, H. P., Amer. J. Physiol., 137, 348 (1942).
Hestrin, S., J. Biol. Chem., 180, 249 (1949).
Mares-Guia, M., and Shaw, E., Abstracts, Sixth International Congress of Biochemistry, 4, 100 (1964).
Salzman, E. W., and Chambers, D. A., Nature, 204, 698 (1964).
Marr, J., Barboriak, J., and Johnson, S. A., Nature, 205, 259 (1965).
Käser-Glanzmann, R., and Lüscher, E. F., Thromb. Diath. Haemorrh., 7, 480 (1962).
Grette, K., Acta Physiol. Scand., 56, suppl. 195 (1962).
Constantine, J. W., Nature, 207, 91 (1965).
Gaarder, A., Jonsen, J., Laland, S., Hellem, A., and Owren, P. A., Nature, 192, 531 (1961).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
COLE, E., KOPPEL, J. & OLWIN, J. Multiple Specificity of Thrombin for Synthetic Substrates. Nature 213, 405–406 (1967). https://doi.org/10.1038/213405a0
Issue date:
DOI: https://doi.org/10.1038/213405a0