Abstract
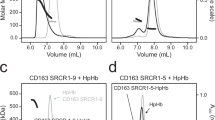
HAPTOGLOBIN, one of the α 2 globulins of human serum, is thought to combine irreversibly with haemoglobin in a molecular ratio of one to one1,2. Two bands are therefore expected after gel electrophoresis of haptoglobin to which haemoglobin has been added in non-saturating proportions, a rapidly migrating band of free haptoglobin and a slower one for the haptoglobin–haemoglobin complex. Reported results are different. In agar gel electrophoresis, a single zone occurs with a mobility that decreases with increasing haemoglobin concentration. Two possible explanations are offered by Wieme3. Complex formation may be reversible, making it possible for a few haemoglobin molecules to interact with all of the haptoglobin molecules, resulting in a migration rate intermediate between free and fully saturated haptoglobin. More probably, the haptoglobin–haemoglobiri complex has residual binding capacity for other haptoglobin molecules. In this way the influence of one haemoglobin molecule extends over several molecules of haptoglobin. Both explanations are incompatible with the commonly held ideas about the character of the haptoglobin–haemoglobin complex. In starch gel, undersaturated haptoglobin type 1-1 (ref. 4) shows a complex with a mobility intermediate between that of the uncombined and the fully saturated protein. Laurell5 suggests that haptoglobin may pick up a half molecule of haemoglobin in these conditions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Jayle, M. F., and Boussier, G., Exposés Ann. Biochim. Méd., 17, 157 (1955).
Jayle, M. F., and Moretti, J., in Progress in Hematology (edit. by Tocantino, L. M.), 3, 342 (Grune and Stratton, New York, 1962).
Wieme, R. J., Agar Gel Electrophoresis, 229 (Elsevier, Amsterdam, 1965).
Smithies, O., and Walker, N. F., Nature, 178, 694 (1956).
Laurell, C. B., Clin. Chim. Acta, 4, 79 (1959).
Raymond, S., and Nakamichi, M., Anal. Biochem., 7, 225 (1964).
Peacock, A. C., Bunting, S. L., and Queen, K. G., Science, 147, 1451 (1965).
Raymond, S., Miles, J. L., and Lee, J. C. J., Science, 151, 346 (1966).
Oster, G. K., Oster, G., and Prati, G., J. Amer. Chem. Soc., 79, 595 (1957).
Connell, G. E., and Shaw, R. W., Canad. J. Biochem., 39, 1013 (1961).
Pintera, J., Collection Czecoslov. Chem. Commun., 32, 876 (1967).
Zwaan, J., Anal. Biochem., 21, 155 (1967).
Benesch, R. E., Benesch, R., and MacDuff, G., Proc. US Nat. Acad. Sci., 54, 535 (1965).
Antonini, E., Wyman, J., Bucci, E., Fronticelli, C., and Rossi-Fanelli, A., J. Mol. Biol., 4, 368 (1962).
Antonini, E., Science, 158, 1417 (1967).
Nagel, R. L., and Ranney, H. M., Science, 144, 1014 (1964).
Nagel, R. L., and Gibson, Q. H., J. Biol. Chem., 242, 3428 (1967).
Ingram, V. M., The Hemoglobins in Genetics and Evolution, 6 (Columbia University Press, New York, 1963).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
ZWAAN, J., MAKI, T. Haptoglobin–Haemoglobin Complexes studied by Polyacrylamide Gel Electrophoresis. Nature 218, 476–478 (1968). https://doi.org/10.1038/218476a0
Received:
Published:
Issue date:
DOI: https://doi.org/10.1038/218476a0