Abstract
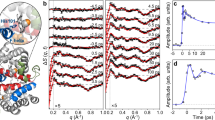
A preliminary analysis of myoglobin proves that neutron diffraction analysis of proteins is feasible and should reveal the position of fixed hydrogen atoms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Moore, F. M., Willis, B. T. M., and Crowfoot Hodgkin, D., Nature, 214, 130 (1967).
Bacon, G. E., Advances in Structure Research by Diffraction Methods, 1 (Interscience, 1964).
MacDonald, A. C., and Sikka, S. K., Acta Cryst. (in the press).
Parrish, R. G., and Kendrew, J. C., Proc. Roy. Soc., A, 238, 305 (1956).
Hamilton, W. C., J. Comp. Physics, 2, 417 (1968).
Watson, H. C., Prog. Stereochem. (1968).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
SCHOENBORN, B. Neutron Diffraction Analysis of Myoglobin. Nature 224, 143–146 (1969). https://doi.org/10.1038/224143a0
Received:
Issue date:
DOI: https://doi.org/10.1038/224143a0
This article is cited by
-
Macromolecular structure phasing by neutron anomalous diffraction
Scientific Reports (2016)
-
Synthesis of 3-(5-bromo-2,3-dimethoxy-phenyl)-[1, 2, 4] oxadiazole analogues and their evaluation as anti-Parkinson′s agents
Medicinal Chemistry Research (2008)
-
The march of structural biology
Nature Reviews Molecular Cell Biology (2002)
-
Neutron diffraction reveals oxygen–histidine hydrogen bond in oxymyoglobin
Nature (1981)