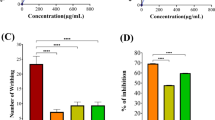
Abstract
MALFORMIN, (cyclo-D-cysteinyl-D-cysteinyl-L-valyl-D-leucyl-L-isoleucyl)1, is produced by the fungus Aspergillus niger van Tiegh., induces severe malformations on stems and petioles of various plants, and causes root curvature2. When 14C-malformin was supplied to cuttings of the bean Phaseolus vulgaris L. most of the 14C was found in the wall fraction and was liberated from a protein-like component in the walls as 14C-malformeic acid (SO3H-malformin) by oxidation3. Previous results indicated that malformin inhibited wall synthesis and altered wall composition4–6. We recently found that malformin is a potent stimulator of plant growth and inhibits several phytochrome- and ethylene-mediated responses. Because some effects of light and ethylene on plant growth have been attributed to an increase in extensin8–11, a hydroxyproline-rich protein in plant walls, it was suggested that malformin may alter the synthesis or function of this wall component7. Because malformin activity is inhibited by reducing agents12 and it reacts with sulphydryl compounds13, involvement of wall sulphydryl groups in the activity of malformin was also proposed7. We report here on the effect of hydroxyproline and proline, amino acids important in the synthesis of extensin, on the growth-stimulating and root-curving activities of malformin.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Bodanszky, M., and Stahl, G. L., Proc. natn. Acad. Sci. U.S.A., 71, 2791–2794 (1974); Bioorg. Chem., 4, 93–105 (1975).
Curtis, R. W., Pl. Physiol., Lancaster, 33, 17–22 (1958); 36, 37–43 (1961); Science, 128, 661–662 (1958).
Ciarlante, D., and Curtis, R. W., in Proc. 8th Int. Conf. Plant Growth Substances, 396–403 (Hirokawa, Tokyo, 1974).
Curtis, R. W., and Kandler, O., Pl. Physiol., Lancaster, 37, 691–695 (1962).
Curtis, R. W., Pl. Cell Physiol., 10, 203–211 (1969).
Izhar, S., Bevington, J., and Curtis, R. W., Pl. Cell Physiol., 10, 687–698 (1969).
John, W. W., and Curtis, R. W., Experientia, 30, 1392–1393 (1974), Pl. Cell Physiol., 16, 719–728 (1975).
Cleland, R., and Karlsnes, A. M., Pl. Physiol., Lancaster, 42, 669–671 (1967).
Ridge, I., and Osborne, D. J., Nature new Biol., 229, 205–208 (1971).
Sadava, D., and Chrispeels, M. J., Devl Biol., 30, 49–55 (1973).
Lamport, D., Adv. bot. Res., 2, 151–218 (1965).
Suda, S., and Curtis, R. W., Pl. Physiol., Lancaster, 39, 904–906 (1964).
Iriuchijima, S., and Curtis, R. W., Phytochemistry 9, 1199–1202 (1970).
Postlethwait, S., and Curtis, R. W., Am. J. Bot., 46, 31–35 (1959).
Steward, F. C., and Pollard, J. K., Nature, 182, 828–832 (1958).
Cleland, R., Pl. Physiol., Lancaster, 42, 271–274 (1967); 42, 1165–1170 (1967).
Cleland, R., and Olson, A. C., Biochemistry, 7, 1745–1751 (1968).
Rendina, G., Experimental Methods in Modern Biochemistry, 324 (Saunders, Philadelphia, 1971).
Chao, H., and Dashek, W. V., Ann. Bot., 37, 95–105 (1973).
Kivirikko, K. I., and Liesmaa, M., Scand. J. clin. Lab. Invest., 11, 128–133 (1959).
Troll, W., and Lindsley, J., J. biol. Chem., 215, 655–660 (1955).
Moore, S., and Stein, W., J. biol. Chem., 176, 367–388 (1948).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
BUCKHOUT, T., CURTIS, R. Inhibition of malformin activity by hydroxyproline and restoration by proline. Nature 260, 435–436 (1976). https://doi.org/10.1038/260435a0
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1038/260435a0