Abstract
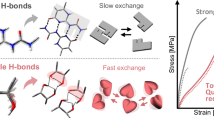
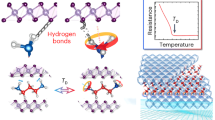
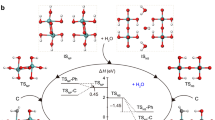
ICE, paper and regenerated cellulose, Nylon (unstretched and lightly stretched), and to a certain extent natural cellulose, lignin and wool are typical examples of hydrogen-bond dominated solids (whose mechanical properties are mostly controlled by the density and characteristics of the hydrogen bond). Previously1 I have shown that for these materials, Young's modulus E is related to N, the effective number of H bonds per cm3 responding to unidirectional stress, by E = kN1/3. For cellulosics k is ∼ 8 × 102 when E is in Pa. Two major rheological characteristics of hydrogen-bond dominated solids are their ready softening by water and their relaxation of stress with time under constant strain. Thus, EW/E0, the ratio of the modulus at w g H2O/g solid to the modulus at w= 0, drops to very small values for paper at saturation. Similarly, Et/E0, the modulus at time t to E at t = 0 in a stress relaxation experiment, drops to small ratios at high values of t. I postulate that both types of loss in E arise from reductions in N, through H-bond dissociation. I find this dissociation can be in one of three modes: I, II and III.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Nissan, A. H., Trans. Faraday Soc., 53, 700–721 (1957); Nature, 175, 424–425 (1955); in Surfaces and Coatings (edit. by Marchessault, R. H., and Skaar, C.), 221–265 (Syracuse University Press, 1967).
Frank, H. S., and Wen, W-Y., Discuss. Faraday Soc., 24, 133–140 (1957).
Starkweather, H. W., Jr, Macromolecules, 8, 476–479 (1975).
Zimm, B. H., J. chem. Phys., 21, 934–935 (1955).
Zimm, B. H., and Lundberg, J. L., J. phys. Chem., 60, 425–428 (1956).
Némethy, G., and Scheraga, H. A., J. chem. Phys., 36, 3382–3400; 3401–3417 (1962).
Higgins, H. G., and Balodis, Y., Fracture (edit. by Osborn, C. J.), 201–220 (University of Melbourne Press, 1965).
Andersson, O., and Berkyto, E., Svensk Papp Tidn., 54, 441–444 (1951).
Benson, R. E., Tappi, 54, 699–703 (1971).
Riemen, W. P., and Kurath, S. K., Tappi, 47, 629–633 (1974).
Brezinski, J. P., thesis, Institute of Paper Chemistry, Appleton, Wisconsin (1955).
Meredith, R., J. Text. Inst., 48, T163–T174 (1957).
Adams, N., J. Text. Inst., 47, T530–T540 (1956).
Quistwater, J. M. R., and Dunell, B. A., J. polym. Sci., 28, 309–318 (1958); J. appl. polym. Sci., 1, 267–271 (1959).
Haughton, P. M., and Sellen, D. B., J. Phys. D., 6, 1998–2011 (1973).
Nissan, A. H., Macromolecules (in the press).
Brunauer, S., Emmett, P. H., and Teller, E., J. Am. chem. Soc., 60, 309 (1938).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
NISSAN, A. Three modes of dissociation of H bonds in hydrogen-bond dominated solids. Nature 263, 759 (1976). https://doi.org/10.1038/263759a0
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1038/263759a0