Abstract
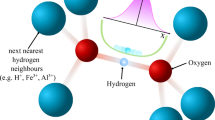
CIRCULAR hydrogen bonds present a new, experimentally demonstrated principle showing how hydration water molecules and hydroxyl groups of macromolecules can cooperate to form a network-like pattern. Quantum chemical calculations show that chain-like H-bonds in the crystal lattice1 are energetically favoured above individual ones2,3. This is due to the cooperative effect, which leads to increased H-bonding activity of an OH-group if it is already accepting or donating an H-bond. These linear structures can close up to form circular arrangements comprising four and more OH-groups4–6. Such circles have actually been described for crystal structures of ice7 and of ice clathrates8, but in these cases the water molecules within the circles are related by crystallographic symmetry elements and are therefore not independent of each other. One should expect to find lattice-independent circular H-bonds in crystal structures of large O—H-rich molecules which co-crystallise with water of hydration, conditions which are satisfied by the cyclodextrin family. The smallest member, α-cyclodextrin (α-CD; cyclohexaamylose), consists of six α(1, 4)-linked glucose molecules and contains six primary and 12 secondary hydroxyl groups ((C6H10O5)6, molecular weight 973). From aqueous solution, α-CD crystallises as hexahydrate, α-CD·6H2O, and this complex, with a total of 120 hydroxyl groups (4 × 18 from α-CD and 4 × 12 from the six H2O) in one unit cell has been studied by X-ray and neutron diffraction methods (ref. 9, and Klar, Hingerty and W.S., unpublished). Two other complexes, a second modification of the hexahydrate (K. Lindner and W.S., unpublished) and α-CD·methanol·4H2O (ref. 10) have been investigated by X rays. As refinement in all cases is around R = 4% for data extending to 0.89 Å resolution, hydrogen atom positions could be assigned. I discuss here results obtained from the X-ray/neutron study of α-CD·6H2O.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Jeffrey, G. A. & Takagi, S. Acct. Chem. Res. 11, 264–270 (1978).
Del Bene, J. E. & Pople, J. A. J. chem. Phys. 52, 4858–4866 (1970); 58, 3605–3608 (1973).
Del Bene, J. E. J. chem. Phys. 55, 4633–4636 (1971).
Karpfen, A., Ladik, J., Russegger, P., Schuster, P. & Suhai, S. Theor. chim. Acta 34, 115–127 (1974).
Kerns, R. C. & Allen, L. C. J. Am. chem. Soc. 100, 6587–6594 (1978).
Schuster, P. in The Hydrogen Bond Vol 1 (eds Schuster, P., Zundel, G. & Sandorfy, C.) 25–164 (North-Holland, Amsterdam, 1976).
Peterson, S. W. & Levy, H. A. Acta crystallogr. 10, 70–76 (1957).
Jeffrey, G. A. & McMullan, R. K. Prog. inorg. Chem. 8, 43–108 (1967).
Manor, P. C. & Saenger, W. Nature 237, 392–393 (1972); J. Am. chem. Soc. 96, 3630–3639 (1974).
Hingerty, B. & Saenger, W. Nature 255, 396–397 (1975); J. Am. chem. Soc. 98, 3357–3365 (1976).
Allinger, W. L. Adv. phys. org. Chem. 13, 17 (1976).
van Etten, R. L., Glowes, G. A., Sebastian, J. F. & Bender, M. L. J. Am. chem. Soc. 89, 3253–3262 (1967).
van Hooidonk, C. & Groos, C. C. Rec. Trav. Chim. Pays-Bas 89, 845–856 (1970).
Frank, H. S. & Wen, W.-Y. Trans. Faraday Soc. 24, 133–140 (1957).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
SAENGER, W. Circular hydrogen bonds. Nature 279, 343–344 (1979). https://doi.org/10.1038/279343a0
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1038/279343a0
This article is cited by
-
A theoretical study on intermolecular hydrogen bonds of isopropanol-water clusters
Theoretical Chemistry Accounts (2022)
-
Structure and dynamics of liquid water
Journal of Structural Chemistry (2006)
-
Coordinated proton tunnelling in a cyclic network of four hydrogen bonds in the solid state
Nature (1999)
-
Crystal structures of dibenzo-14-crown-4 alcohol and diol monohydrates
Journal of Inclusion Phenomena and Molecular Recognition in Chemistry (1995)
-
Structure and some properties of small water clusters
Journal of Structural Chemistry (1994)