Abstract
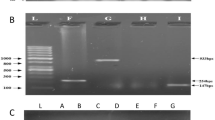
In a variety of mammalian species including humans the newborn young are particularly susceptible to diarrhoeal disease brought about by toxin-producing strains of the common gut bacterium, Escherichia coli1. Two types of enterotoxin have been described2. The first, heat-labile toxin (LT), sharing partial antigenic identity with cholera toxin2,3 is comprised of two subunits having molecular weights of 25,500 and 11,5004 and, like cholera toxin, stimulates adenyl cyclase activity in the gut5. The second type, heat-stable toxin (ST), is a low-molecular weight protein which is excreted into the medium during the growth of ST-elaborating strains. Unlike LT, ST is poorly antigenic and for this reason the identification of ST-producing strains has relied on an assay involving the intragastric injection of infant mice, a test specific for ST6. Although many strains produce both ST and LT, strains producing ST alone are especially common in E. coli diarrhoeal disease of calves7,8, pigs2 and have also been implicated in outbreaks of diarrhoea in humans9,10. Recent evidence suggests that ST activates guanyl cyclase in the gut11,12 and its molecular weight has been estimated to be 4,400–5,100 (ref. 13). We have now applied gene cloning techniques to the isolation of ST genes from two porcine E. coli isolates and report here the identification of the protein product of molecular weight 7,000, synthesised in an in vitro system directed by the cloned DNA template. This in vitro gene product elicits a typical ST enterotoxin response in the infant mouse assay and is most probably synthesised as a 10,000 molecular weight precursor, as might be expected for an exported protein. The gene cloning data also provide evidence that the ST genes and their surrounding transposon structures are probably identical in both porcine and bovine strains of entero-pathogenic E. coli.
This is a preview of subscription content, access via your institution
Access options
Similar content being viewed by others
References
Sack, R. B. A. Rev. Microbiol. 29, 333–353 (1975).
Gyles, C. L. Ann. N.Y. Acad. Sci. 176, 314–322 (1971).
Smith, N. & Sack, R. B. J. infect. Dis. 127, 164–170 (1973).
Dallas, W. S. & Falkow, S. Nature 277, 406–407 (1979).
Evans, D. J., Chen, L. C., Curlin, G. T. & Evans, D. G. Nature new Biol. 236, 137–138 (1972).
Dean, A. G., Ching, Y.-C., Williams, R. C. & Harden, L. B. J. infect. Dis. 125, 407–411
Sivaswamy, G. & Gyles, C. L. Can. J. comp. Med. 40, 241–246 (1976).
Kaeckenbeek, A., Schoenaers, F., Pastoret, P. P. & Josse, M. Ann. Med. Vet. 121, 55–57 (1977).
Ryder, H. W. et al. New Engl. J. Med. 295, 849–853 (1976).
Kudoh, Y., Zen-Yoji, H., Matsushita, S., Sakai, S. & Maruyama, T. Microbiol. Immun. 21, 175–178 (1977).
Hughes, J. M., Murad, F., Chang, B. & Guerrant, R. L. Nature 271, 755–756 (1978).
Field, M., Graf, L. H., Laird, W. J. & Smith, P. L. Proc. natn. Acad. Sci. U.S.A. 75, 2800–2804 (1978).
Alderete, J. F. & Robertson, D. C. Infect. Immun. 19, 1021–1030 (1978).
Smith, H. W. & Halls, S. J. gen. Microbiol. 52, 319–334 (1968).
Gyles, C. L., So, M. & Falkow, S. J. infect. Dis. 130, 40–49 (1974).
Smith, H. W. Soc. appl. Bact. Symp. 227–242 (1976).
So, M., Heffron, F. & McCarthy, B. J. Nature 277, 453–456 (1979).
Harford, N. et al. (in preparation).
Chang, A. C. Y. & Cohen, S. N. J. Bact. 134, 1141–1156 (1978).
Bolivar, F. et al. Gene 2, 95–113 (1977).
Fuchs, E. Eur. J. Biochem. 63, 15–22 (1976).
Blobel, G. & Dobberstein, B. J. Cell. Biol. 67, 835–851 (1975).
Sarvas, M. et al. (in preparation).
Laemmli, U. K. Nature 227, 680–685 (1970).
Laskey, R. A. & Mills, A. D. Eur. J. Biochem. 56, 335–341 (1975).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lathe, R., Hirth, P., Wilde, M. et al. Cell-free synthesis of enterotoxin of E. coli from a cloned gene. Nature 284, 473–474 (1980). https://doi.org/10.1038/284473a0
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1038/284473a0
This article is cited by
-
Identification of enterotoxigenicEscherichia coli using alkaline phosphatase-labeled synthetic oligodeoxyribonucleotide probes
European Journal of Clinical Microbiology & Infectious Diseases (1988)
-
AIDS virus env Protein Expressed from a Recombinant Vaccinia Virus
Nature Biotechnology (1986)
-
Active γ-carboxylated human factor IX expressed using recombinant DNA techniques
Nature (1985)
-
Expression of rabies virus glycoprotein from a recombinant vaccinia virus
Nature (1984)