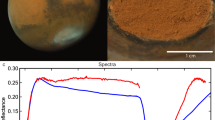
Abstract
The red colour of Mars has long been attributed to the occurrence of ferric oxides on its surface and in atmospheric dust storms. Such a belief has been accentuated by results of Viking Lander experiments on the surface of Mars, which suggested the presence of maghaemite, γ-Fe2O3 (refs 1, 2). This dark brown, ferromagnetic iron (III) oxide phase not only conforms with the colour and magnetic properties of dust on the martian surface, but suggests that maghaemite acted as a catalyst in some of the biological experiments performed in situ on Mars3. Many of the physical properties and chemical reactions attributed to maghaemite, however, are also displayed by δ-FeOOH, an oxide hydroxide polymorph of ferric iron4,5. On Earth, δ-FeOOH occurs as the mineral feroxyhyte in gleyed soils and in submarine manganese nodule deposits6. Some of the properties and paragenetic relationships of feroxyhyte are described here and it is suggested that δ-FeOOH is a prime candidate for the red-brown ferromagnetic phase coating the surface of Mars.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Hargraves, R. B., Collinson, D. W., Arvidson, R. E. & Spitzer, C. R. J. geophys. Res. 82, 4547 (1977).
Toulmin, P. III et al. J. geophys. Res. 82, 4625 (1977).
Oyama, V. I. & Berdahl, B. J. J. geophys. Res. 82, 4669 (1977).
Burns, R. G. & Burns, V. M. in Marine Manganese Deposits (ed. Glasby, G. P.) 212 (Elsevier, Amsterdam, 1977).
Murray, J. W. in Marine Minerals Ch. 2 (ed. Burns, R. G.) 47 (Mineralology Society of America Monograph 6, 1979).
Chukhrov, F. V., Zvyagin, B. B., Yermilova, L. P. & Gorshkov, A. I. Miner. Deposita 11, 24 (1976).
Okamoto, S. J. Am. ceram. Soc. 51, 594 (1968).
Atkinson, R. J. Aust. J. Chem. 29, 2149 (1976).
Coey, J. M. D. & Khalafalla, D. Phys. Stat. Sol. A 11, 229 (1972).
Feitknecht, W., Haeni, H. & Dvorak, V. in Proc. 6th int. Symp. Reactivity of Solids, 237 (1969).
Houck, J. R., Pollack, J. B., Sagan, C., Schaack, D. & Decker, J. A. Jr Icarus 18, 470 (1973).
Sara, J. Chem. Listy. 63, 112 (1969).
Burns, R. G. & Burns, V. M. in The Sea Vol. 7 (ed. Emiliani, C.) (Wiley, New York, in the press).
Burns, R. G. & Burns, V. M. Nature 255, 130 (1975).
Burns, V. M. & Burns, R. G. Earth planet. Sci. Lett. 39, 341 (1978).
Huguenin, R. L., Prinn, R. G. & Maderazzo, M. Icarus 32, 270 (1977).
Klein, H. P. Icarus 34, 666 (1978).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Burns, R. Does feroxyhyte occur on the surface of Mars?. Nature 285, 647 (1980). https://doi.org/10.1038/285647a0
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1038/285647a0
This article is cited by
-
Viking Biology Experiments: Lessons Learned and the Role of Ecology in Future Mars Life-Detection Experiments
Space Science Reviews (2008)
-
Mössbauer spectroscopy on the surface of Mars. Why?
Hyperfine Interactions (1992)
-
Feroxyhyte on Mars?
Nature (1980)
-
Feroxyhyte on Mars? (reply)
Nature (1980)