Abstract
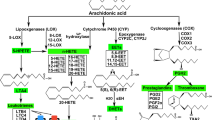
The arachidonic acid released from cellular phospholipids of specifically stimulated platelets and leukocytes is oxygenated enzymatically by two major pathways1–5. A complex cyclo-oxygenase converts some of the free arachidonic acid to labile endoperoxides that are transformed to prostaglandins, thromboxanes and prostacyclin (PGI2). Lipoxygenases convert part of the arachidonic acid to unstable hydroperoxy-eicosatetraenoic acids (OOHETEs) that are transformed to monohydroxy-eicosatetraenoic acids (HETEs), oligohydroxy-eicosatetraenoic or -eicosatrienoic acids such as di-HETEs and tri-HETEs, and, in some instances, more complex humoral mediators, including slow-reacting substances5–9. Both the nature of the HETEs and the ratio of the HETEs to the cyclo-oxygenase products are specific characteristics of each type of cell2,5. In human neutrophils, the sum of the lipoxygenase products 5-HETE, 11-HETE and 5,12-di-HETE substantially exceeds the total amount of PGE2 and other cyclo-oxygenase metabolites that are generated concurrently3,4, and the endogenous lipoxygenase products regulate neutrophil function. The present data indicate that vitamin E (α-tocopherol) bidirectionally modulates the activity of the lipoxygenase pathway of human neutrophils in vitro. Normal plasma concentrations of α-tocopherol enhance the lipoxygenation of arachidonic acid, whereas higher concentrations of α-tocopherol exert a suppressive effect that is consistent with its role as a hydroperoxide scavenger.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Samuelsson, B., Granstrom, E., Green, K., Hamberg, M. & Hammarstrom, S. A. Rev. Biochem. 44, 669–695 (1975).
Nugteren, D. H. Biochim. biophys. Acta 380, 299–307 (1975).
Goldstein, I. M. et al. J. exp. Med. 148, 787–792 (1978).
Stenson, W. F. & Parker, C. W. J. clin. Invest. 64, 1457–1465 (1979).
Borgeat, P., Hamberg, M. & Samuelsson, B. J. biol. Chem. 251, 7816–7820 (1976).
Borgeat, P. & Samuelsson, B. Proc. natn. Acad. Sci. U.S.A. 76, 3213–3217 (1979).
Jones, R. L., Kerry, P. J., Poyser, N. L., Walker, I. C. & Wilson, N. H. Prostaglandins 16, 583–589 (1978).
Murphy, R. C., Hammarström, S. & Samuelsson, B. Proc. natn. Acad. Sci. U.S.A. 76, 4275–4280 (1979).
Morris, H. R., Taylor, G. W., Piper, P. J., Sanhoun, M. W. & Tippins, J. R. Prostaglandins 19, 185–201 (1980).
Goetzl, E. J., Woods, J. M. & Gorman, R. R. J. clin. Invest. 59, 179–183 (1977).
Goetzl, E. J. & Sun, F. F. J. exp. Med. 150, 406–411 (1979).
Goetzl, E. J., Brash, A. R., Tauber, A. I., Oates, J. A. & Hubbard, W. C. Immunology 39, 141–151 (1980).
Goetzl, E. J., Hill, H. R. & Gorman, R. R. Prostaglandins 19, 71–85 (1980).
Goetzl, E. J., Weller, P. F. & Sun, F. F. J. Immun. 124, 926–933 (1980).
Goetzl, E. J. Immunology 40, 709–726 (1980).
Tappel, A. L. Vitam. Horm. 20, 493–510 (1962).
Tappel, A. L. Ann. N.Y. Acad. Sci. 203, 12–28 (1972).
Hoekstra, W. G. Fedn Proc. 34, 2083–2089 (1975).
Combs, G. F. Jr., Noguchi, T. & Scott, M. L. Fedn Proc. 34, 2090–2095 (1975).
Harris, R. E., Boxer, L. A. & Baehner, R. L. Blood 55, 338–343 (1980).
Chan, A. C., Hegarty, P. V. J. & Allen, C. E. J. Nutr. 110, 74–81 (1980).
Chan, A. C., Allen, C. E. & Hegarty, P. V. J. J. Nutr. 110, 66–73 (1980).
Steiner, M. & Anastasi, J. J. clin. Invest. 57, 732–737 (1976).
Hatam, L. J. & Kayder, H. J. J. Lipid Res. 20, 639–645 (1979).
Kilian, P. L. et al. Biochem. biophys. Res. Commun. 93, 409–414 (1980).
Mueller, P. & Rudin, D. O. J. theor. Biol. 18, 222–258 (1968).
Boeynaems, J. M., Oates, J. A. & Hubbard, W. C. Prostaglandins 19, 87–91 (1980).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Goetzl, E. Vitamin E modulates the lipoxygenation of arachidonic acid in leukocytes. Nature 288, 183–185 (1980). https://doi.org/10.1038/288183a0
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1038/288183a0
This article is cited by
-
Endogenous metabolites of vitamin E limit inflammation by targeting 5-lipoxygenase
Nature Communications (2018)
-
Effects of vitamin E ingestion on plasma and urinary risk factors for calcium oxalate urolithiasis in two population groups having different stone-risk profiles: evidence of different physiological handling mechanisms
Urological Research (2012)
-
Exacerbation of dextran sulfate sodium-induced colitis by dietary iron supplementation: role of NF-κB
International Journal of Colorectal Disease (2006)
-
Vitamin E and vitamin C inhibit arachidonate-induced aggregation of human peripheral blood leukocytesin vitro
Agents and Actions (1986)
-
Stimulation by lipoxygenase products of superoxide anion production in FMLP‐treated neutrophils
Lipids (1985)