Abstract
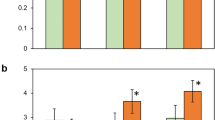
Halophytes are plants which tolerate high concentrations of electrolytes of which NaCl is normally the dominating salt. Compartmentation of the electrolytes within the cytoplasm has not been unequivocally resolved1. Several soluble enzymes from higher plant halophytes showed similar sensitivity to salinity when tested in vitro as did those from glycophytes2. Also, isolated mitochondria from halophytes and glycophytes showed similar inhibition of oxidative phosphorylation by NaCl in vitro3. On the other hand, chloride (plus the accompanying cations) has been shown to be accumulated in the chloroplasts of Limonium vulgare4–6, and it is also an essential cofactor in photosynthetic O2 evolution7–10. In the only report available on the effects of salt on chloroplasts from a halophyte11, it was demonstrated that differences exist in the response to salts of thylakoids from halophytic or mesophytic plants. I show here that thylakoids from mature leaves of the grey mangrove, Avicennia marina, require high chloride levels for photosynthetic electron transport around photosystem II and discuss the possibility of chloride accumulation into the chloroplast as an adaptational mechanism for salt tolerance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Flowers, T. J., Troke, P. F. & Yeo, A. R. A. Rev. Pl. Physiol. 28, 89–121 (1977).
Greenway, H. & Osmond, C. B. Pl. Physiol. 49, 256–259 (1972).
Flowers, T. J. J. exp. Bot. 101, 101–110 (1974).
Larkum, A. W. D. Nature 218, 447–449 (1968).
Larkum, A. W. D. & Hill, A. E. Biochim. biophys. Acta 203, 133–138 (1970).
Ziegler, H. & Lüttge, U. Planta 74, 1–17 (1967).
Warburg, O. & Lüttgens, W. Naturwissenschaften 32, 301 (1944).
Gorham, P. R. & Clendenning, K. A. Archs Biochem. Biophys. 37, 199–233 (1952).
Punnett, T. Pl. Physiol. 34, 283–289 (1959).
Hind, G., Nakatani, H. Y. & Izawa, S. Biochim. biophys. Acta 172, 277–289 (1969).
Wignarajah, K. & Baker, N. R. Physiol. Pl. 51, 387–393 (1981).
Baker, N. R. Pl. Physiol. 62, 889–893 (1978).
Böhme, H., Reimer, S. & Trebst, A. Z. Naturforsch. b26, 341–352 (1971).
Satoh, K., Katoh, S. & Takamiya, A. Pl. Cell Physiol. 11, 453–466 (1970).
Kelley, P. M. & Izawa, S. Biochim. biophys. Acta 502, 198–210 (1978).
Heath, R. L. & Hind, G. Biochim. biophys. Acta 172, 290–299 (1969).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Critchley, C. Stimulation of photosynthetic electron transport in a salt-tolerant plant by high chloride concentrations. Nature 298, 483–485 (1982). https://doi.org/10.1038/298483a0
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/298483a0
This article is cited by
-
Salinity induced alterations in photosynthetic and oxidative regulation are ameliorated as a function of salt secretion
Journal of Plant Research (2021)
-
Mangrove distribution in relation to seasonal water salinity and ion compartmentation: a field study along a freshwater-dominated river
Hydrobiologia (2020)
-
Mangroves: obligate or facultative halophytes? A review
Trees (2011)
-
Interaction of electron acceptors with thylakoids from halophytic and non-halophytic species
Photosynthesis Research (1988)
-
Protection of photosynthetic O2 evolution against heat inactivation: the role of chloride, pH and coupling status
Photosynthesis Research (1988)