Abstract
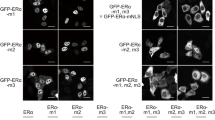
According to the current model of steroid hormone action oestrogen is thought to bind to its receptor in the cytoplasm of target cells1,2 and the oestrogen–receptor complex is then translocated into the nucleus2–4. This model is based on evidence obtained in homogenized cell preparations in which free receptor is associated with the cytosol, whereas steroid-bound receptor is associated with the nuclear fraction. Some data suggest, however, that the unfilled receptor may reside in the nucleus, and that cytosolic localization represents an extraction artefact. We have now reinvestigated the subcellular distribution of unfilled oestrogen receptor using cytochalasin B-induced enucleation to obtain cytoplast and nucleoplast fractions from receptor-containing GH3 cells derived from rat pituitary tumours. We found that cytoplasts prepared from GH3 cells contain little oestrogen-binding activity and that most of the unfilled oestrogen receptors are associated with the nuclear fraction. We therefore suggest that the standard model is in error and that the unoccupied receptor is nuclear in the intact cell.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Noteboom, W. D. & Gorski, J. Archs Biochem. Biophys. 111, 559–568 (1965).
Gorski, J., Toft, D., Shyamala, G., Smith, D. & Notides, A. Recent Prog. Horm. Res. 24, 45–80 (1968).
Jensen, E. V. et al. Proc. natn. Acad. Sci. U.S.A. 59, 632–638 (1968).
Stumpf, W. E. Endocrinology 83, 777–782 (1968).
Gorski, J. & Raker, B. Endocrinology 93, 1212–1216 (1973).
Sheridan, P. J., Buchanin, J. M. & Anselmo, V. C. Nature 282, 579–582 (1979).
Siiteri, P. K. et al. Adv. exp. Med. Biol. 36, 97–112 (1973).
Jordan, V. C., Gosden, B. & Tate, A. C. Endocrinology 112, abstr. 407 (1983).
Muldoon, T. J. biol. Chem. 255, 1358–1366 (1980).
Walters, M., Hunziker, W. & Norman, A. J. biol. Chem. 255, 6799–6805 (1980).
Samuels, H. & Tsai, J. Proc. natn. Acad. Sci. U.S.A. 70, 3488–3492 (1973).
Zava, D. & McGuire, W. J. biol. Chem. 252, 3703–3708 (1977).
Edwards, D., Martin, P. M., Horwitz, K. B., Chamness, G. C. & McGuire, W. L. Expl Cell Res. 127, 197–213 (1980).
Morel, G. et al. Expl Cell Res. 132, 249–257 (1981).
Jensen, E., Greene, G., Closs, L., DeSombre, E. & Nadji, M. Recent Prog. Horm. Res. 38, 1–40 (1982).
Raam, S., Nemeth, E., Tamura, H., O'Briain, D. & J. Eur. J. Cancer clin. Oncol. 18, 1–12 (1982).
King, W. & Greene, G. Nature (in the press).
Tashjian, A., Bancroft, F. & Levine, L. J. Cell Biol. 47, 61–70 (1970).
Tashjian, A., & Hoyt, R. in Molecular Genetics and Developmental Biology (ed. Sussman, M. 353–387 (Prentice-Hall Englewood Cliffs, 1972).
Haug, E., Naess, O. & Gautvik, K. M. Molec. cell Endocr. 12, 81–95 (1978).
Noteboom, W. D., Durham, J. B. & Mitra, R. J. Steroid Biochem. 16, 633–638 (1982).
Haug, E. & Gautvik, K. M. Endocrinology 99, 1482–1489 (1976).
Tate, A. C., Lieberman, M. & Jordan, V. C. J. Steroid Biochem. (in the press, 1984).
Bossart, W., Loeffler, H. & Bienz, K. Exp Cell Res. 96, 360–366 (1975).
Bradford, M. Analyt. Biochem. 72, 248–254 (1976).
Burton, K. Biochem. J. 62, 315–323 (1956).
Labarca, C. & Paigen, K. Analyt. Biochem. 102, 344–352 (1980).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Welshons, W., Lieberman, M. & Gorski, J. Nuclear localization of unoccupied oestrogen receptors. Nature 307, 747–749 (1984). https://doi.org/10.1038/307747a0
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1038/307747a0
This article is cited by
-
Mating changes the subcellular distribution and the functionality of estrogen receptors in the rat oviduct
Reproductive Biology and Endocrinology (2009)
-
Membrane Estrogen Receptors Acting Through Metabotropic Glutamate Receptors: An Emerging Mechanism of Estrogen Action in Brain
Molecular Neurobiology (2008)