Abstract
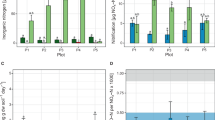
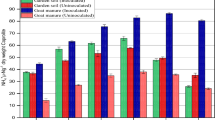
The high resistance of organic nitrogen complexes in soil to microbial attack is of significance to crop production, N balance of soils, geochemical and pedological processes, and environ mental quality1–3. Several theories have been given to account for this phenomenon. An important theory indicates the stabilization of nitrogen through the formation of nitrogenous polymers during the reaction of nitrogenous substances with polyphenols1–3. It is well known that the formation of nitrogenous polymers from nitrogenous substances and polyphenols is accelerated in the presence of phenol oxidase enzymes1–8 and oxidants such as Ag2O (ref. 9). However, the promoting effects of soil inorganic components on the formation of organic N complexes have received little attention. Mn(IV) oxides are common in soil10. Furthermore, the effect of Mn(IV) oxides on the oxidative polymerization of hydroquinone is much greater than that of various other inorganic components such as short-range ordered Fe(III), Al and Si oxides11, primary minerals12 and clay minerals13 which are commonly present in soil environments. We report here that Mn(IV) oxide (birnessite), which is common in natural environments, greatly promotes the abiotic formation of nitrogenous polymers in hydroquinone–glycine systems in the common pH range (4–8) of soils. The findings indicate that Mn(IV) oxides merit close attention in the abiotic formation of organic N complexes from nitrogenous substances and polyphenols and the subsequent turnover of N in soil and the associated emvironments.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Bremner, J. M. in Soil Biochemistry Vol. 1 (eds McLaren, A. D. & Peterson, G. H.) 19–66 (Dekker, New York, 1967).
Parsons, J. W. & Tinsley, J. in Soil Components Vol. 1 (ed. Gieseking, J. E.) 263–304 (Springer, New York, 1975).
Stevenson, F. J. in Nitrogen in Agricultural Soils (ed. Stevenson, F. J.) 1–42, 67–122 (American Society of Agronomy, Crop Science Society of America, Soil Science Society of America, Madison, 1982).
Haider, K., Frederick, L. R. & Flaig, W. Pl. Soil 22, 49–64 (1965).
Flaig, W., Beutelspacher, H. & Rietz, E. in Soil Components Vol. 1 (ed. Gieseking, J. E.) 1–211 (Springer, New York, 1975).
Verma, L., Martin, J. P. & Haider, K. Soil Sci. Soc. Am. J. 39, 279–284 (1975).
Haider, K., Martin, J. P. & Filip, Z. in Soil Biochemistry Vol. 4 (eds Paul, E. A. & McLaren, A. D.) 195–244 (Dekker, New York, 1975).
Martin, J. P. & Haider, K. Soil Sci. Soc. Am. J. 44, 983–988 (1980).
Ladd, J. N. & Butler, J. H. A. in Rep. FAO/IAEA tech. Meet. 143–150 (Pergamon, Oxford, 1966).
McKenzie, R. M. Miner. Mag. 38, 493–502 (1971).
Shindo, H. & Huang, P. M. Nature 298, 363–365 (1982).
Shindo, H. & Huang, P. M. Soil Sci. (submitted).
Kumada, K. Chemistry of Soil Organic Matter 2nd edn (in Japanese, Japan Scientific Society Press, Tokyo, 1981).
Harborne, J. B. & Simmonds, N. W. in Biochemistry of Phenolic Compounds (ed. Harborne, J. B.) 77–127 (Academic, London, 1964).
Mason, H. S. Nature 175, 771–772 (1955).
Weast, R. C. (ed.) CRC Handbook of Chemistry and Physics 59th edn (CRC Press, Boca Raton, Florida, 1978).
Kuwahara, M., Shindo, N. & Munakata, K. J. agric. Chem. Soc. Japan (in Japanese) 44, 169–174 (1970).
Bremner, J. M. in Methods of Soil Analysis Pt 2 (eds Black, C. A., Evans, D. D., White, J. L., Ensminger, L. E. & Clark, R. E.) 1149–1237 (American Society of Agronomy, Madison, 1965).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shindo, H., Huang, P. Significance of Mn(IV) oxide in abiotic formation of organic nitrogen complexes in natural environments. Nature 308, 57–58 (1984). https://doi.org/10.1038/308057a0
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1038/308057a0
This article is cited by
-
Evolution of humic substances in polymerization of polyphenol and amino acid based on non-destructive characterization
Frontiers of Environmental Science & Engineering (2021)
-
Abiotic contribution to phenol oxidase activity across a manganese gradient in tropical forest soils
Biogeochemistry (2021)
-
Role of ferric oxide in abiotic humification enhancement of organic matter
Journal of Material Cycles and Waste Management (2017)
-
Aging promotes todorokite formation from layered manganese oxide at near-surface conditions
Journal of Soils and Sediments (2010)
-
Is separating resource competition from allelopathy realistic?
The Botanical Review (1997)