Abstract
Study design: It has been previously demonstrated that sustained nonpatterned electric stimulation of the posterior lumbar spinal cord from the epidural space can induce stepping-like movements in subjects with chronic, complete spinal cord injury. In the present paper, we explore physiologically related components of electromyographic (EMG) recordings during the induced stepping-like activity.
Objectives: To examine mechanisms underlying the stepping-like movements activated by electrical epidural stimulation of posterior lumbar cord structures.
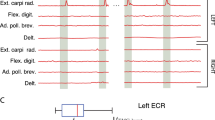
Materials and methods: The study is based on the assessment of epidural stimulation to control spasticity by simultaneous recordings of the electromyographic activity of quadriceps, hamstrings, tibialis anterior, and triceps surae. We examined induced muscle responses to stimulation frequencies of 2.2–50 Hz in 10 subjects classified as having a motor complete spinal cord injury (ASIA A and B). We evaluated stimulus-triggered time windows 50 ms in length from the original EMG traces. Stimulus-evoked compound muscle action potentials (CMAPs) were analyzed with reference to latency, amplitude, and shape.
Results: Epidural stimulation of the posterior lumbosacral cord recruited lower limb muscles in a segmental-selective way, which was characteristic for posterior root stimulation. A 2.2 Hz stimulation elicited stimulus-coupled CMAPs of short latency which were approximately half that of phasic stretch reflex latencies for the respective muscle groups. EMG amplitudes were stimulus-strength dependent. Stimulation at 5–15 and 25–50 Hz elicited sustained tonic and rhythmic activity, respectively, and initiated lower limb extension or stepping-like movements representing different levels of muscle synergies. All EMG responses, even during burst-style phases were composed of separate stimulus-triggered CMAPs with characteristic amplitude modulations. During burst-style phases, a significant increase of CMAP latencies by about 10 ms was observed.
Conclusion: The muscle activity evoked by epidural lumbar cord stimulation as described in the present study was initiated within the posterior roots. These posterior roots muscle reflex responses (PRMRRs) to 2.2 Hz stimulation were routed through monosynaptic pathways. Sustained stimulation at 5–50 Hz engaged central spinal PRMRR components. We propose that repeated volleys delivered to the lumbar cord via the posterior roots can effectively modify the central state of spinal circuits by temporarily combining them into functional units generating integrated motor behavior of sustained extension and rhythmic flexion/extension movements. This study opens the possibility for developing neuroprostheses for activation of inherent spinal networks involved in generating functional synergistic movements using a single electrode implanted in a localized and stable region.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Grillner S . Control of locomotion in bipeds, tetrapods, and fish. In: Brooks VB (ed). Handbook of Physiology. The Nervous System. Motor Control, Section 1, Vol. II. Am. Physiol. Soc: Washington, DC, 1981, pp 1179–1236.
Rossignol S . Neural control of stereotypic limb movements. In: Rowell LB, Shepherd JT (eds). Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems, Am Physiol Soc: Bethesda, MD, 1996, Section 12, pp 173–216.
Illis LS . Is there a central pattern generator in man? Paraplegia 1995; 33: 239–240.
Bussel B, Roby-Brami A, Neris OR, Yakovleff A . Evidence for a spinal stepping generator in man. Paraplegia 1996; 34: 91–92.
Roby-Brami A, Bussel B . Long-latency spinal reflex in man after flexor reflex afferent stimulation. Brain 1987; 110: 707–725.
Calancie B, Needham-Shropshire B, Jacobs P, Willer K, Zych G, Green BA . Involuntary stepping after chronic spinal cord injury. Evidence for a central rhythm generator for locomotion in man. Brain 1994; 117: 1143–1159.
Gurfinkel VS, Levik YS, Kazennikov OV, Selionov VA . Locomotor-like movements evoked by leg muscle vibration in humans. Eur J Neurosci 1998; 10: 1608–1612.
Dimitrijevic MM, Dimitrijevic MR, Illis LS, Nakajima K, Sharkey PC, Sherwood AM . Spinal cord stimulation for the control of spasticity in patients with chronic spinal cord injury: I. Clinical observations. Cent Nerv Syst Trauma 1986; 3: 129–144.
Dimitrijevic MR, Illis LS, Nakajima K, Sharkey PC, Sherwood AM . Spinal cord stimulation for the control of spasticity in patients with chronic spinal cord injury: II. Neurophysiologic observations. Cent Nerv Syst Trauma 1986; 3: 145–152.
Barolat G, Singh-Sahni K, Staas WE, Shatin D, Ketcik B, Allen K . Epidural spinal cord stimulation in the management of spasms in spinal cord injury. A prospective study. Stereotact Funct Neurosurg 1995; 64: 153–164.
Pinter MM, Gerstenbrand F, Dimitrijevic MR . Epidural electrical stimulation of posterior structures of the human lumbosacral cord: 3. Control of spasticity. Spinal Cord 2000; 38: 524–531.
Rosenfeld JE, McKay WB, Halter JA, Pollo F, Dimitrijevic MR . Evidence of a pattern generator in paralyzed subjects with spinal cord injury during spinal cord stimulation. Soc Neurosci Abstr 1995; 21: 688.
Gerasimenko Y, McKay B, Sherwood A, Dimitrijevic MR . Stepping movements in paraplegic patients induced by spinal cord stimulation. Soc Neurosci Abstr 1996; 22: 1372.
Dimitrijevic MR, Gerasimenko Y, Pinter MM . Evidence for a spinal central pattern generator in humans. In: Kiehn O, Harris-Warrik RM, Jordan LM, Hultborn H, Kudo N (eds). Neural Mechanisms for Generating Locomotor Activity. Ann N Y Acad Sci, Vol. 860. New York Academy of Sciences: New York, 1998, pp 360–376.
Sherwood AM, McKay WB, Dimitrijevic MR . Motor control after spinal cord injury: assessment using surface EMG. Muscle Nerve 1996; 19: 966–979.
Lehmkuhl D, Dimitrijevic MR, Renouf F . Electrophysiological characteristics of lumbosacral evoked potentials in patients with established spinal cord injury. Electroencephalogr Clin Neurophysiol 1984; 59: 142–155.
Beric A . Stability of lumbosacral somatosensory evoked potentials in a long-term follow-up. Muscle Nerve 1988; 11: 621–626.
Struijk JJ, Holsheimer J, Boom HB . Excitation of dorsal root fibers in spinal cord stimulation: a theoretical study. IEEE Trans Biomed Eng 1993; 40: 632–639.
Holsheimer J . Which neuronal elements are activated directly by spinal cord stimulation. Neuromodulation 2002; 5: 25–31.
Murg M, Binder H, Dimitrijevic MR . Epidural electric stimulation of posterior structures of the human lumbar spinal cord: 1. Muscle twitches – a functional method to define the site of stimulation. Spinal Cord 2000; 38: 394–402.
Lang J . Morphologie und funktionelle Anatomie der Lendenwirbelsäule und des benachbarten Nervensystems – 1. Rückenmark. In: Hohmann D, Kügelgen B, Liebig K, Schirmer M (eds). Neuroorthopädie 2 – Lendenwirbelsäulenerkrankungen mit Beteiligung des Nervensystems, Springer: Berlin, 1984, pp 3–9.
Jilge B, Minassian K, Dimitrijevic MR . Electrical stimulation of the human lumbar cord can elicit standing parallel extension of paralysed lower limbs after spinal cord injury. Proceedings of the World Congress on Neuroinformatics, Vienna, Austria, 2001, Part II, pp 281–283.
Jilge B, Minassian K, Rattay F, Pinter MM, Gerstenbrand F, Binder H, Dimitrijevic MR . Initiating extension of the lower limbs in subjects with complete spinal cord injury by epidural lumbar cord stimulation. Exp Brain Res 2004; 154: 308–326.
Dimitrijevic MR, Minassian K, Jilge B, Rattay F . Initiation of standing and locomotion like movements in complete SCI subjects ‘by mimicking’ brain stem control of lumbar network with spinal cord stimulation. Proceedings of the fourth International Symposium on Experimental Spinal Cord Injury Repair and Regeneration, Brescia, Italy, 2002, pp 25–27.
Dimitrijevic MR . Neurophysiological evaluation and epidural stimulation in chronic spinal cord injury patients. In: Reier PJ, Bunge RP, Kao CC (eds). Spinal Cord Reconstruction, Raven Press: New York, 1983, pp 465–473.
Hunter JP, Ashby P . Segmental effects of epidural spinal cord stimulation in humans. J Physiol 1994; 474: 407–419.
He J, Barolat G, Holsheimer J, Struijk JJ . Perception threshold and electrode position for spinal cord stimulation. Pain 1994; 59: 55–63.
Barolat G, Massaro F, He J, Zeme S, Ketcik B . Mapping of sensory responses to epidural stimulation of the intraspinal neural structures in man. J Neurosurg 1993; 78: 233–239.
Guru K, Mailis A, Ashby P, Vanderlinden G . Postsynaptic potentials in motoneurons caused by spinal cord stimulation in humans. Electroencephalogr Clin Neurophysiol 1987; 66: 275–280.
Wall EJ, Cohen MS, Abitbol JJ, Garfin SR . Organization of intrathecal nerve roots at the level of the conus medullaris. J Bone Joint Surg 1990; 72: 1495–1499.
Geddes LA, Baker LE . The specific resistance of biological material – a compendium of data for the biomedical engineer and physiologist. Med Biol Eng 1967; 5: 271–293.
Coburn B . A theoretical study of epidural electrical stimulation of the spinal cord – Part II: Effects on long myelinated fibers. IEEE Trans Biomed Eng 1985; 32: 978–986.
Rattay F, Minassian K, Dimitrijevic MR . Epidural electrical stimulation of posterior structures of the human lumbosacral cord: 2. quantitative analysis by computer modeling. Spinal Cord 2000; 38: 473–489.
Maccabee PJ et al. A new method using neuromagnetic stimulation to measure conduction time within the cauda equina. Electroencephalogr Clin Neurophysiol 1996; 101: 153–166.
Troni W, Bianco C, Moja MC, Dotta M . Improved methodology for lumbosacral nerve root stimulation. Muscle Nerve 1996; 19: 595–604.
Lloyd DPC . Reflex action in relation to pattern and peripheral source of afferent stimulation. J Neurophysiol 1943; 16: 111–120.
Magladery JW, Porter WE, Park AM, Teasdall RD . Electrophysiological studies of nerve and reflex activity in normal man. Bull John Hopkins Hosp 1951; 88: 499–519.
Minassian K, Rattay F, Dimitrijevic MR . Features of the reflex responses of the human lumbar cord isolated from the brain but during externally controlled locomotor activity. Proceedings of the World Congress on Neuroinformatics. Vienna, Austria 2001, Part II, pp 267–268.
Minassian K et al. Effective spinal cord stimulation (SCS) train for evoking stepping locomotor movement of paralyzed human lower limbs due to SCI elicits a late response additionally to the early monosynaptic response. Soc Neurosci Abstr 2001; 27, Program no. 935.12.
Minassian K et al. Effective spinal cord stimulation (SCS) for evoking stepping movement of paralyzed human lower limbs: study of posterior root muscle reflex responses. Proceedings of the Seventh Annual Conference of the IFESS, Ljubljana, Slovenia, 2002, pp 167–169.
Zehr EP, Stein RB . What functions do reflexes serve during human locomotion? Prog Neurobiol 1999; 58: 185–205.
Capaday C . The special nature of human walking and its neural control. Trends Neurosci 2002; 25: 370–376.
Clarac F, Cattaert D, Le Ray D . Central control components of a ‘simple’ stretch reflex. Trends Neurosci 2000; 23: 199–208.
Pearson KG . Proprioceptive regulation of locomotion. Curr Opin Neurobiol 1995; 5: 786–791.
Pearson KG, Ramirez J-M . Sensory modulation of pattern generating circuits. In: Stein PSG, Grillner S, Selverston AI, Stuart DG (eds). Neurons, Networks and Motor Behavior, MIT Press: Cambridge, USA, 1999, pp 257–267.
Hultborn H . State-dependent modulation of sensory feedback. J Physiol 2001; 533: 5–13.
McCrea DA . Spinal circuitry of sensorimotor control of locomotion. J Physiol 2001; 533: 41–50.
Lundberg A . Multisensory control of spinal reflex pathways. Prog Brain Res 1979; 50: 11–28.
Jankowska E . Spinal interneuronal systems: identification, multifunctional character and reconfigurations in mammals. J Physiol 2001; 533: 31–40.
Hultborn H et al. How do we approach the locomotor network in the mammalian spinal cord?. In: Kiehn O, Harris-Warrik RM, Jordan LM, Hultborn H, Kudo N (eds). Neural Mechanisms for Generating Locomotor Activity, Ann N Y Acad Sci, Vol. 860 New York Academy of Sciences: New York, 1998, pp 70–82.
Burke RE, Degtyarenko AM, Simon ES . Patterns of locomotor drive to motoneurons and last-order interneurons: clues to the structure of the CPG. J Neurophysiol 2001; 86: 447–462.
Dimitrijevic MR, Gerasimenko Y, Pinter MM . Effect of reduced afferent input on lumbar CPG in spinal cord injury subjects. Soc Neurosci Abstr 1998; 24, Program No. 654.23.
Edgley SA . Organisation of inputs to spinal interneurone populations. J Physiol 2001; 533: 51–56.
Grillner S, Wallen P, Hill R, Cangiano L, El Manira A . Ion channels of importance for the locomotor pattern generation in the lamprey brainstem-spinal cord. J Physiol 2001; 533: 23–30.
Acknowledgements
We thank Ms Preinfalk, Ms Auer, and Ms Alesch for their excellent technical support. We gratefully acknowledge the funding supports from the Austrian Ministry of Transport, Innovation and Technology, the Austrian Science Fund (FWF research project P15469), and the Kent Waldrep National Paralysis Foundation in Addison, Texas, USA.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Minassian, K., Jilge, B., Rattay, F. et al. Stepping-like movements in humans with complete spinal cord injury induced by epidural stimulation of the lumbar cord: electromyographic study of compound muscle action potentials. Spinal Cord 42, 401–416 (2004). https://doi.org/10.1038/sj.sc.3101615
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.sc.3101615
Keywords
This article is cited by
-
Tapping into the human spinal locomotor centres with transspinal stimulation
Scientific Reports (2024)
-
Soleus H-reflex amplitude modulation during walking remains physiological during transspinal stimulation in humans
Experimental Brain Research (2024)
-
Beyond treatment of chronic pain: a scoping review about epidural electrical spinal cord stimulation to restore sensorimotor and autonomic function after spinal cord injury
Neurological Research and Practice (2023)
-
A case study of percutaneous epidural stimulation to enable motor control in two men after spinal cord injury
Nature Communications (2023)
-
Rare phenomena of central rhythm and pattern generation in a case of complete spinal cord injury
Nature Communications (2023)