Abstract
Study design:
A rat model of spinal cord injury was used to test the hypothesis that Nogo-A monoclonal antibody (NEP1-40) promotes morphologic and functional recoveries of injured spinal cord.
Objective:
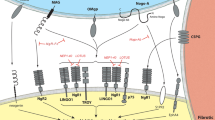
Nogo-A is a myelin-associated neurite outgrowth inhibitory protein, which blocks elongation nerve fibers and limits neuronal regeneration after central nervous system injury.
Methods:
Forty-four rats were utilized and allocated into control (vehicle) and NEP1-40-treated groups. In all animals, the spinal cord was hemi-transected at Th-10 and phosphate-buffered saline solution was immediately applied on the injured area in the control group. NEP1-40 solution was immediately applied on the hemi-transected area in the treatment group. Each group was subdivided into three subgroups according to the postsurgical day of killing (3, 8 and 21 days). The spinal cords were removed for analysis.
Results:
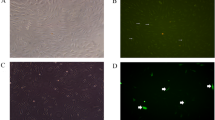
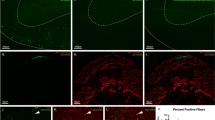
Motor scores in the NEP1-40-treated groups were significantly higher than those in the vehicle groups both at 8 and 21 days post injury. Immunohistochemical staining for pan-cadherin, a marker of neuronal cell adhesion and axonal sprouting, revealed a significant increase in staining in the NEP1-40 treatment group at 8 and 21 days post injury. Transmission electron microscopical evaluation revealed degeneration of the myelin and loss of cytoarchitectural organization in the axons of controls. Better preservation and normal histologic features were observed in the NEP1-40-treated groups.
Conclusion:
We have demonstrated improved preservation of injured axons and significant pan-cadherin expression after NEP1-40 treatment after the spinal cord injury. Inhibition of Nogo-A may improve the capacity for neuronal regeneration after spinal cord injury.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Brook GA et al. Attempted endogenous tissue repair following experimental spinal cord injury in the rat: involvement of cell adhesion molecules L1 and NCAM? Eur J Neurosci 2000; 12: 3224–3238.
Buss A, Schwab ME . Sequential loss of myelin proteins during Wallerian degeneration in the rat spinal cord. Glia 2003; 42: 424–432.
Chen MS et al. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature 2000; 403: 434–439.
Oertle T, Schwab ME . Nogo and its paRTNers. Trends Cell Biol 2003; 13: 187–194.
GrandPre T, Li S, Strittmatter SM . Nogo-66 receptor antagonist peptide promotes axonal regeneration. Nature 2002; 417: 547–551.
Barton WA et al. Structure and axon outgrowth inhibitor binding of the Nogo-66 receptor and related proteins. EMBO J 2003; 22: 3291–3302.
Skaper SD, Moore SE, Walsh FS . Cell signalling cascades regulating neuronal growth-promoting and inhibitory cues. Prog Neurobiol 2001; 65: 593–608.
Massaro AR . The role of NCAM in remyelination. Neurol Sci 2002; 22: 429–435.
Takeichi M, Abe K . Synaptic contact dynamics controlled by cadherin and catenins. Trends Cell Biol 2005; 15: 216–221.
Okamura K et al. Cadherin activity is required for activity-induced spine remodeling. J Cell Biol 2004; 167: 961–972.
Arai M, Goto T, Seichi A, Nakamura K . Effects of antithrombin III on spinal cord-evoked potentials and functional recovery after spinal cord injury in rats. Spine 2004; 29: 405–412.
Soy O et al. Time–level relationship for nitric oxide and the protective effects of aminoguanidine in experimental spinal cord injury. Acta Neurochir (Wien) 2004; 146: 1329–1335; discussion 1335–1336.
Colak A KA, Barut S, Kokturk S, Akyildiz AI, Tasyurekli M . Neuroprotection and functional recovery after application of the caspase-9 inhibitor z-LEHD-fmk in a rat model of traumatic spinal cord injury. J Neurosurg Spine 2005; 2: 327–334.
Ferretti P, Zhang F, O'Neill P . Changes in spinal cord regenerative ability through phylogenesis and development: lessons to be learnt. Dev Dyn 2003; 226: 245–256.
Fouad K, Dietz V, Schwab ME . Improving axonal growth and functional recovery after experimental spinal cord injury by neutralizing myelin associated inhibitors. Brain Res Brain Res Rev 2001; 36: 204–212.
Schwab ME, Thoenen H . Dissociated neurons regenerate into sciatic but not optic nerve explants in culture irrespective of neurotrophic factors. J Neurosci 1985; 5: 2415–2423.
Bregman BS, Kunkel-Bagden E, Schnell L, Dai HN, Gao D, Schwab ME . Recovery from spinal cord injury mediated by antibodies to neurite growth inhibitors. Nature 1995; 378: 498–501.
Keirstead HS, Hasan SJ, Muir GD, Steeves JD . Suppression of the onset of myelination extends the permissive period for the functional repair of embryonic spinal cord. Proc Natl Acad Sci USA 1992; 89: 11664–11668.
Brosamle C, Huber AB, Fiedler M, Skerra A, Schwab ME . Regeneration of lesioned corticospinal tract fibers in the adult rat induced by a recombinant, humanized IN-1 antibody fragment. J Neurosci 2000; 20: 8061–8068.
Thornton MR, Mantovani C, Birchall MA, Terenghi G . Quantification of N-CAM and N-cadherin expression in axotomized and crushed rat sciatic nerve. J Anat 2005; 206: 69–78.
Acknowledgements
This study was supported by Baskent University Research Fund, Ankara, Turkey.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Atalay, B., Bavbek, M., Cekinmez, M. et al. Antibodies neutralizing Nogo-A increase pan-cadherin expression and motor recovery following spinal cord injury in rats. Spinal Cord 45, 780–786 (2007). https://doi.org/10.1038/sj.sc.3102113
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.sc.3102113
Keywords
This article is cited by
-
The Nogo-66 receptor family in the intact and diseased CNS
Cell and Tissue Research (2012)