Abstract
Study design:
Immunohistochemical investigation in control and lesioned human spinal cords.
Objectives:
To assess the spatial and temporal expression patterns of transforming growth factor-β1 and -β2 (TGF-β1 and TGF-β2) in the human spinal cord after traumatic injury.
Setting:
Germany, Aachen, Aachen University Hospital.
Methods:
Sections from human spinal cords from 4 control patients and from 14 patients who died at different time points after traumatic spinal cord injury (SCI) were investigated immunohistochemically.
Results:
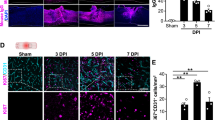
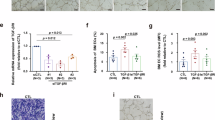
In control cases, TGF-β1 was confined to occasional blood vessels, intravascular monocytes and some motoneurons, whereas TGF-β2 was only found in intravascular monocytes. After traumatic SCI, TGF-β1 immunoreactivity was dramatically upregulated by 2 days after injury (the earliest survival time investigated) and was detected within neurons, astrocytes and invading macrophages. The staining was most intense over the first weeks after injury but gradually declined by 1 year. TGF-β2 immunoreactivity was first detected 24 days after injury. It was located in macrophages and astrocytes and remained elevated for up to 1 year. In white matter tracts undergoing Wallerian degeneration, there was no induction of either isoform.
Conclusion:
The early induction of TGF-β1 at the point of SCI suggests a role in the acute inflammatory response and formation of the glial scar, while the later induction of TGF-β2 may indicate a role in the maintenance of the scar. Neither of these TGF-β isoforms appears to contribute to the astrocytic scar formation in nerve fibre tracts undergoing Wallerian degeneration.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Hausmann ON . Post-traumatic inflammation following spinal cord injury. Spinal Cord 2003; 41: 369–378.
Schwab ME, Bartholdi D . Degeneration and regeneration of axons in the lesioned spinal cord. Physiol Rev 1996; 76: 319–370.
Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV . Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci 2004; 24: 2143–2155.
Ashcroft GS . Bidirectional regulation of macrophage function by TGF-beta. Microbes Infect 1999; 1: 1275–1282.
Branton MH, Kopp JB . TGF-beta and fibrosis. Microbes Infect 1999; 1: 1349–1365.
Flanders KC, Ren RF, Lippa CF . Transforming growth factor-betas in neurodegenerative disease. Prog Neurobiol 1998; 54: 71–85.
De Groot CJ, Montagne L, Barten AD, Sminia P, Van Der Valk P . Expression of transforming growth factor (TGF)-beta1, -beta2, and -beta3 isoforms and TGF-beta type I and type II receptors in multiple sclerosis lesions and human adult astrocyte cultures. J Neuropathol Exp Neurol 1999; 58: 174–187.
Lagord C, Berry M, Logan A . Expression of TGF-β2 but not TGF-β1 correlates with the deposition of scar tissue in the lesioned spinal cord. Mol Cell Neurosci 2002; 20: 69–82.
Peress NS, Perillo E . Differential expression of TGF-beta 1, 2 and 3 isotypes in Alzheimer's disease: a comparative immunohistochemical study with cerebral infarction, aged human and mouse control brains. J Neuropathol Exp Neurol 1995; 54: 802–811.
McTigue DM, Popovich PG, Morgan TE, Stokes BT . Localization of transforming growth factor-beta1 and receptor mRNA after experimental spinal cord injury. Exp Neurol 2000; 163: 220–230.
Yang L, Blumbergs PC, Jones NR, Manavis J, Sarvestani GT, Ghabriel MN . Early expression and cellular localization of proinflammatory cytokines interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in human traumatic spinal cord injury. Spine 2004; 29: 966–971.
Flanders KC, Lippa CF, Smith TW, Pollen DA, Sporn MB . Altered expression of transforming growth factor-beta in Alzheimer's disease. Neurology 1995; 45: 1561–1569.
O'Brien MF, Lenke LG, Lou J, Bridwell KH, Joyce ME . Astrocyte response and transforming growth factor-beta localization in acute spinal cord injury. Spine 1994; 19: 2321–2329.
Rogister B, Delree P, Leprince P, Martin D, Sadzot C, Malgrange B et al. Transforming growth factor beta as a neuronoglial signal during peripheral nervous system response to injury. J Neurosci Res 1993; 34: 32–43.
Unsicker K, Krieglstein K . TGF-betas and their roles in the regulation of neuron survival. Adv Exp Med Biol 2002; 513: 353–374.
Flanders KC, Ludecke G, Engels S, Cissel DS, Roberts AB, Kondaiah P et al. Localization and actions of transforming growth factor-betas in the embryonic nervous system. Development 1991; 113: 183–191.
Mangasser-Stephan K, Gressner AM . Molecular and functional aspects of latent transforming growth factor-beta binding protein: just a masking protein? Cell Tissue Res 1999; 297: 363–370.
Roberts AB, McCune BK, Sporn MB . TGF-beta: regulation of extracellular matrix. Kidney Int 1992; 41: 557–559.
Streit WJ, Semple-Rowland SL, Hurley SD, Miller RC, Popovich PG, Stokes BT . Cytokine mRNA profiles in contused spinal cord and axotomized facial nucleus suggest a beneficial role for inflammation and gliosis. Exp Neurol 1998; 152: 74–87.
Bunge RP, Puckett WR, Hiester ED . Observations on the pathology of human spinal cord injury, with emphasis on the astrocyte response to penetrating injuries. Adv Neurol 1997; 72: 305–315.
Logan A, Green J, Hunter A, Jackson R, Berry M . Inhibition of glial scarring in the injured rat brain by a recombinant human monoclonal antibody to transforming growth factor-beta2. Eur J Neurosci 1999; 11: 2367–2374.
Moon LD, Fawcett JW . Reduction in CNS scar formation without concomitant increase in axon regeneration following treatment of adult rat brain with a combination of antibodies to TGF beta1 and beta2. Eur J Neurosci 2001; 14: 1667–1677.
Buss A, Brook GA, Kakulas B, Martin D, Franzen R, Schoenen J et al. Gradual loss of myelin and formation of an astrocytic scar during Wallerian degeneration in the human spinal cord. Brain 2004; 127: 34–44.
Acknowledgements
We thank S Lecouturier for excellent technical assistance. This work was supported by a grant from the Deutsche Stiftung Querschnittslähmung (DSQ).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Buss, A., Pech, K., Kakulas, B. et al. TGF-β1 and TGF-β2 expression after traumatic human spinal cord injury. Spinal Cord 46, 364–371 (2008). https://doi.org/10.1038/sj.sc.3102148
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.sc.3102148
Keywords
This article is cited by
-
Newly developed TGF-β2 knock down transgenic mouse lines express TGF-β2 differently and its distribution in multiple tissues varies
BMC Biochemistry (2013)
-
The glial scar in spinal cord injury and repair
Neuroscience Bulletin (2013)
-
Transforming growth factor β1-induced astrocyte migration is mediated in part by activating 5-lipoxygenase and cysteinyl leukotriene receptor 1
Journal of Neuroinflammation (2012)
-
Dopamine and α-synuclein dysfunction in Smad3 null mice
Molecular Neurodegeneration (2011)
-
Oligodendrocyte Fate after Spinal Cord Injury
Neurotherapeutics (2011)