Abstract
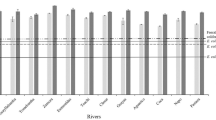
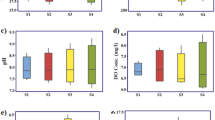
Reliable measurements of the concentrations of dissolved trace metals in rivers are needed for calculating oceanic chemical mass balances, understanding continental weathering and freshwater chemistry, and evaluating anthropogenic chemical perturbations. For dissolved zinc, it has been suggested1 that typical river concentrations are as high as 450 nmol kg−1. However, it has also been proposed2 that most published values are incorrect and the true value of dissolved zinc may be 2 orders of magnitude lower. Because zinc is one of the most commonly used metals in the industrial world, the sampling and analysis of this element in natural waters is particularly prone to contamination2. We present here the dissolved zinc concentrations from various rivers draining both pristine and industrially influenced environments. These samples were collected and analysed by methods that have yielded reliable oceanic data for other metals3. The data indicate that in relatively undisturbed systems dissolved zinc concentrations are typically only 10−9–10−8 mol kg−1, with some dependence of concentration on pH. In industrially influenced river systems, however, zinc concentrations can be 1–2 orders of magnitude higher.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Martin, J.-M. & Meybeck, M. Mar. Chem. 7, 173–206 (1979).
Martin, J. H., Knauer, G. A. & Flegal, A. R. in Zinc in the Environment (ed. Nriagu, J. O.) Part 1, 193–197 (Wiley, New York, 1980).
Boyle, E. A., Huested, S. S. & Jones, S. P. J. geophys. Res. 86, 8048–8066 (1981).
Measures, C. I. & Edmond, J. M. Earth planet. Sci. Lett. 66, 101–110 (1983).
Bowen, H. J. M. Environmental Chemistry of the Elements (Academic, New York, 1979).
Bourg, A. C. M. in Trace Metals in Sea Water (eds Wong, C. S., Boyle, E., Bruland, K. W., Burton, J. D. & Goldberg, E. D.) 195–208 (Plenum, New York, 1983).
Stallard, R. F. thesis, MIT-WHOI, (1980).
Boyle, E. A. in Copper in the Environment (ed. Nriagu, J. O.) Part 1, 77–88 (Wiley, New York, 1979).
Mead, R. H. et al. Nature 278, 161–163 (1979).
Kurian, G. T. World Data (World Almanac, New York, 1983).
Duinker, J. C. and Nolting, R. F. Neth. J. Sea Res. 12, 205–223 (1978).
Salomans, W. & Forstner, U. Metals in the Hydrocycle (Springer, Berlin, 1984).
Tramontano, J. M. & Church, T. M. Trans. Am. Geophys. Un. 64, 1030 (1983).
Bruland, K. W. in Chemical Oceanography (eds Riley, J. P. & Chester, R.) Vol. 8, 157–220 (Academic, London, 1983).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shiller, A., Boyle, E. Dissolved zinc in rivers. Nature 317, 49–52 (1985). https://doi.org/10.1038/317049a0
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1038/317049a0
This article is cited by
-
Rare Earth Elements Around the Barakah Nuclear Power Plant, UAE
Natural Resources Research (2020)
-
Regionalized aquatic ecotoxicity characterization factor for zinc emitted to soil accounting for speciation and the transfer through groundwater
The International Journal of Life Cycle Assessment (2019)
-
Trace element distribution and risk assessment in South Indian surface waterways
International Journal of Environmental Science and Technology (2017)
-
Concentrations of select dissolved trace elements and anthropogenic organic compounds in the Mississippi River and major tributaries during the summer of 2012 and 2013
Environmental Monitoring and Assessment (2017)
-
Characteristics and source identification of dissolved trace elements in the Jinshui River of the South Qinling Mts., China
Environmental Science and Pollution Research (2015)