Abstract
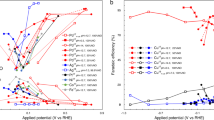
 Since Chatt and co-workers first reported that the protolysis of the dinitrogen complex cis-[W(N2)2(PMe2Ph)4] gives ammonia1, cis-[W(N2)2(PMe2Ph)4]H+→ 2NH3 + N2 + degradation products (1) there has existed the engaging possibility that a cyclic conversion of molecular nitrogen to ammonia might be achieved at room temperature and pressure using mediators possessing an {MP4}-core, M = Mo or W. As a means of circumventing oxidative degradation of the core, coupling protonation with electronation is clearly attractive2. We describe here a system in which this has been achieved for the first time and which forms the basis of an ammonia producing cycle, scheme I in Fig. 1.
Since Chatt and co-workers first reported that the protolysis of the dinitrogen complex cis-[W(N2)2(PMe2Ph)4] gives ammonia1, cis-[W(N2)2(PMe2Ph)4]H+→ 2NH3 + N2 + degradation products (1) there has existed the engaging possibility that a cyclic conversion of molecular nitrogen to ammonia might be achieved at room temperature and pressure using mediators possessing an {MP4}-core, M = Mo or W. As a means of circumventing oxidative degradation of the core, coupling protonation with electronation is clearly attractive2. We describe here a system in which this has been achieved for the first time and which forms the basis of an ammonia producing cycle, scheme I in Fig. 1.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Chatt, J., Pearman, A. J. & Richards, R. L. Nature 253, 39–40 (1975).
Pickett, C. J. Proc. Chromium, Molybdenum and Tungsten Conf. (eds Dilworth, J. R. & Lappert, M. F.) L29 (Royal Society of Chemistry, London, 1983).
Henderson, R. A., Leigh, G. J. & Pickett, C. J. Adv. inorg. Chem. Radiochem. 27, 197–292 (1983).
Al-Salih, T. I. & Pickett, C. J. J. C. S. Dalton, 1255–1264 (1985).
Christou, G., Hageman, R. V. & Holm, R. H. J. Am. chem. Soc. 102, 7600–7601 (1980).
Amatore, C. et al. J. Am. chem. Soc. 107, 1815–1824 (1985).
Pickett, C. J. & Leigh, G. J. J.C.S. chem. Commun., 1033–1035 (1981).
Hussain, W., Leigh, G. J. & Pickett, C. J. J.C.S. chem. Commun., 747–748 (1982).
Lowe, D. J. & Thorneley, R. N. F. Biochem. J. 224, 877–909 (1984).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pickett, C., Talarmin, J. Electrosynthesis of ammonia. Nature 317, 652–653 (1985). https://doi.org/10.1038/317652a0
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1038/317652a0
This article is cited by
-
Advances in electrochemical transformation of N2 using molecular catalysts
Science China Chemistry (2023)
-
Two catalysts are better than one
Nature Energy (2022)
-
Tandem electrocatalytic N2 fixation via proton-coupled electron transfer
Nature (2022)
-
Single-atom catalysts based on two-dimensional metalloporphyrin monolayers for ammonia synthesis under ambient conditions
Nano Research (2022)
-
Over 56.55% Faradaic efficiency of ambient ammonia synthesis enabled by positively shifting the reaction potential
Nature Communications (2019)