Abstract
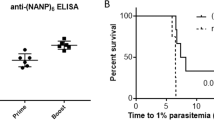
A 12 ammo-acid synthetic peptide (NANP)3 comprising the immunodominant epitope of Plasmodium falciparum circum-sporozoite protein was conjugated to tetanus toxoid (TT), adjuvan-ted with aluminium hydroxide, and administered intramuscularly in three doses at monthly intervals to 35 healthy males as a malaria vaccine. No significant adverse reactions were noted, with mild soreness at the injection site the only common symptom. Serocon-versions against NANP occurred in 53% and 71% of recipients of 100 or 160 μg, respectively, measured by enzyme-linked immunosorbent assay (ELIS A). Most ELISA-positive sera reacted with sporozoites by indirect immunofluorescence (IFA). Three vaccinees with the highest ELISA and IFA titres and four unim-munized controls were challenged with P. falciparum sporozoites introduced via the bites of infective Anopheles mosquitoes. Blood stage parasites were detected in all controls by 10 days (mean 8.5 days, range 7–10). In contrast, the two vaccinees who became infected did not manifest parasitaemia until day 11 and the third vaccinee showed neither parasites nor symptoms during the 29 day observation period. This first synthetic peptide parenteral vaccine against a communicable disease tested in man is safe and stimulates biologically active antibodies. These observations encourage the development of improved vaccine formulations which, by enhancing immunogenicity, may lead to practical vaccines to assist in the control of falciparum malaria.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
1. WHO Technical Report Series, No 735, 1986 (WHO Expert Committe on Malaria: eighteenth report). 2. Miller, L. H. et al. Science 234, 1349–1356 (1986). 3. Clyde, D. F., Most, H., McCarthy, V. C. & Vanderberg, J. P. Am. J. med. Sci. 266, 169–177 (1973). 4. Clyde, D. F., McCarthy, V. C., Miller, R. M. & Woodward, W. E. Am. J. trop. Med. Hyg. 24, 397–401 (1975). 5. Rieckmann, K. H., Beaudoin, R. L., Cassels, J. S. & Sell, K. W. Bull. Wld Hlth Org. 57, (Suppl. 1), 261–265 (1979). 6. Nussenzweig, V. & Nussenzweig, R. S. Cell 42, 401–403 (1985). 7. Zavala, F., Cochrane, A. H., Nardin, E. H., Nussenzweig, R. S. & Nussensweig, V. J. exp. Med. 157, 1947–1957 (1983). 8. Dame, J. B. et al. Science 225, 593–599 (1984). 9. Enea, V. et al. Science 225, 628–630 (1984). 10. Zavala, F. et al. Science 228, 1436–1440 (1985). 11. Young, J. F. et al. Science 228, 958–962 (1985). 12. Barany, G. & Merrifield, R. B. The Peptides: Analysis, Synthesis, Biology Vol. 2 (Academic, New York, 1980). 13. Vanderberg, J., Nussenzweig, R. S. & Most, H. Mil. Med. 134, (Suppl.). 1183–1190 (1969). 14. Nardin, E. H., Nussenzweig, R. S., McGregor, I. A. & Bryan, J. H. Science 206, 597–599 (1979). 15. Levine, M. M. et al. J. din. Invest. 79, 888–902 (1986). 16. Burkot, T. R., Williams, J. L. & Schneider, I. Trans. R. Soc. trop. Med. Hyg. 78,339–341 (1984). 17. Lelijveld, J. & Kortman, H. Bull. Wld Hlth Org. 42, 477–479 (1970). 18. Jeffery, G. M., Young, M. D., Burgess, R. W. & Eyles, D. E. Annals trop. Med. Parasit. 53, 51–58 (1959). 19. Boyd, M. F. Symposium on Human Malaria Pub. 15 (American Association for the Advancement of Science, Washington DC, 1941). 20. Nardin, E. H. et al. J. exp. Med. 156, 20–30 (1982). 21. Schmidt, L. H., Fradkin, R., Genther, C. S., Rossan, R. N., Squires, W. & Hughes, H. B. Am. J. trop. Med. Hyg. 31, (Suppl.), 609–703 (1982). 22. Nussenzweig, R. S. Expl Parasit. 21, 224–231 (1967). 23. Zavala, F. et al. J. exp. Med. (submitted). 24. Herzenberg, L. A., Tokuhis, T. & Herzenberg, L. Nature 285, 665–667 (1980). 25. Schutz, M. P., Leclerc, C., Audibert, F. & Chedid, L. /. Immun. 135, 2319–2322 (1985). 26. Sarin, V. K., Kent, S. B. H., Tarn, J. P. & Merrifield, R. B. Analyt. Biochem. 117, 147–157 (1981). 27. Nardin, E. H., Gwadz, R. W. & Nussenzweig, R. S. Bull. Wld Hlth Org. 57 (Suppl. 1), 211–217 (1979). 28. Ballou, R. S. et al. Lancet i, 1277–1281 (1987).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Herrington, D., Clyde, D., Losonsky, G. et al. Safety and immunogenicity in man of a synthetic peptide malaria vaccine against Plasmodium falciparum sporozoites. Nature 328, 257–259 (1987). https://doi.org/10.1038/328257a0
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1038/328257a0
This article is cited by
-
A VLP for validation of the Plasmodium falciparum circumsporozoite protein junctional epitope for vaccine development
npj Vaccines (2021)
-
Epitope-coated polymer particles elicit neutralising antibodies against Plasmodium falciparum sporozoites
npj Vaccines (2021)
-
Identification of a neutralizing epitope within minor repeat region of Plasmodium falciparum CS protein
npj Vaccines (2021)
-
Structural and biophysical correlation of anti-NANP antibodies with in vivo protection against P. falciparum
Nature Communications (2021)
-
Discovery of four new B-cell protective epitopes for malaria using Q beta virus-like particle as platform
npj Vaccines (2020)