Abstract
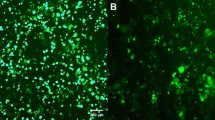
Efficient gene transduction in cardiomyocytes is a task that can be accomplished only by viral vectors. Up to now, the most commonly used vectors for this purpose have been adenoviral-derived ones. Recently, it has been demonstrated that lentiviral vectors can transduce growth-arrested cells, such as hematopoietic stem cells. Moreover, a modified form of lentiviral vector (the ‘advanced’ generation), containing an mRNA-stabilizer sequence and a nuclear import sequence, has been shown to significantly improve gene transduction in growth-arrested cells as compared to the third-generation vector. Therefore, we tested whether the ‘advanced’ generation lentivirus is capable of infecting and transducing cardiomyocytes both in vitro and in vivo, comparing efficacy in vitro against the third-generation of the same vector. Here we report that ‘advanced’ generation lentiviral vectors infected most (>80%) cardiomyocytes in culture, as demonstrated by immunofluorescence and FACS analyses: in contrast the percentage of cardiomyocytes infected by third-generation lentivirus was three- to four-fold lower. Moreover, ‘advanced’ generation lentivirus was also capable of infecting and inducing stable gene expression in adult myocardium in vivo. Thus, ‘advanced’ generation lentiviral vectors can be used for both in vitro and in vivo gene expression studies in the cardiomyocyte.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hajjar RJ, del Monte F, Matsui T, Rosenzweig A . Prospects for gene therapy for heart failure. Circ Res 2000; 86: 616–621.
Svensson EC et al. Efficient and stable transduction of cardiomyocytes after intramyocardial injection or intracoronary perfusion with recombinant adeno-associated virus vectors. Circulation 1999; 99: 201–205.
Kay MA, Glorioso JC, Naldini L . Viral vectors for gene therapy: the art of turning infectious agents into vehicles of therapeutics. Nat Med 2001; 7: 33–40.
Zennou V et al. HIV-1 genome nuclear import is mediated by a central DNA flap. Cell 2000; 101: 173–185.
Follenzi A et al. Gene transfer by lentiviral vectors is limited by nuclear translocation and rescued by HIV-1 pol sequences. Nat Genet 2000; 25: 217–222.
Mochizuki H et al. High-titer human immunodeficiency virus type 1-based vector systems for gene delivery into nondividing cells. J Virol 1998; 72: 8873–8883.
Sakoda T, Kasahara N, Hamamori Y, Kedes L . A high-titer lentiviral production system mediates efficient transduction of differentiated cells including beating cardiac myocytes. J Mol Cell Cardiol 1999; 31: 2037–2047.
Naldini L et al. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci USA 1996; 93: 11382–11388.
Sen A et al. Terminally differentiated neonatal rat myocardial cells proliferate and maintain specific differentiated functions following expression of SV40 large T antigen. J Biol Chem 1988; 263: 19132–19136.
De Luca A et al. Characterization of caveolae from rat heart: localization of postreceptor signal transduction molecules and their rearrangement after norepinephrine stimulation. J Cell Biochem 2000; 77: 529–539.
Condorelli G et al. Cardiomyocytes induce endothelial cells to trans-differentiate into cardiac muscle: implications for myocardium regeneration. Proc Natl Acad Sci USA 2001; 98: 10733–10738.
Dull T et al. A third generation lentivirus vector with a conditional packaging system. J Virol 1998; 71: 8463–8471.
Hajjar RJ et al. Modulation of ventricular function through gene transfer in vivo. Proc Natl Acad Sci USA 1998; 95: 5251–5256.
Ikeda Y et al. Restoration of deficient membrane proteins in the cardiomyopathic hamster by in vivo cardiac gene transfer. Circulation 2002; 105: 502–508.
Acknowledgements
The following sources of support are acknowledged: American Heart Association (GLC), Italian Association for Cancer Research (GLC), Fondi 1% Ministero della Sanita'-Italy (GC, LN and GLC), Telethon Association (GC and LN), European Community (GC) and Progetto Terapia Tumori Italy-USA (GLC)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bonci, D., Cittadini, A., Latronico, M. et al. ‘Advanced’ generation lentiviruses as efficient vectors for cardiomyocyte gene transduction in vitro and in vivo. Gene Ther 10, 630–636 (2003). https://doi.org/10.1038/sj.gt.3301936
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.gt.3301936
Keywords
This article is cited by
-
Ultrasound-targeted microbubble destruction (UTMD)-mediated miR-150-5p attenuates oxygen and glucose deprivation-induced cardiomyocyte injury by inhibiting TTC5 expression
Molecular Biology Reports (2022)
-
Non-viral vector based gene transfection with human induced pluripotent stem cells derived cardiomyocytes
Scientific Reports (2019)
-
TAZ is required for metastatic activity and chemoresistance of breast cancer stem cells
Oncogene (2015)
-
Lentiviral vector–based insertional mutagenesis identifies genes associated with liver cancer
Nature Methods (2013)
-
BTG2 loss and miR-21 upregulation contribute to prostate cell transformation by inducing luminal markers expression and epithelial–mesenchymal transition
Oncogene (2013)