Abstract
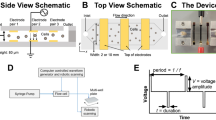
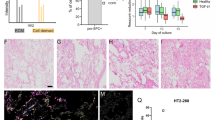
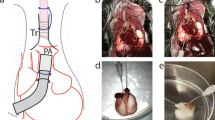
To increase the levels of pulmonary gene transfer by nonviral vectors, we have adopted electroporation protocols for use in the lung. A volume of 100–200 μl of purified plasmid DNA suspended in saline was instilled into the lungs of anesthetized mice. Plasmids expressed luciferase, or β-galactosidase under control of the CMV immediate-early promoter and enhancer. Immediately following delivery, a series of eight square wave electric pulses of 10 ms duration each at an optimal field strength of 200 V/cm were administered to the animals using 10 mm Tweezertrodes (Genetronics, San Diego, CA, USA). The electrodes were placed on either side of the chest, which had been wetted with 70% ethanol. The animals recovered and survived with no apparent trauma until the experiments were terminated at the desired times, between 1 and 7 days post-treatment. Gene expression was detected by 1 day postelectroporation and peaked between 2 and 5 days. By 7 days, expression was back to baseline. By contrast, essentially no gene expression was detected in the absence of electric pulses. Using a β-galactosidase-expressing plasmid, the distribution of gene expression appeared to be concentrated in the periphery of the lung, but was also present throughout the parenchyma. The primary cell types expressing gene product include alveolar type I and type II epithelial cells. No inflammation or lung injury was detected histologically or by cytokine measurements in lungs at either 1 or 24 h following electroporation treatment. These results provide evidence that electroporation is a safe and effective means for introducing naked DNA into the lung and form the basis for future studies on targeted pulmonary gene therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Albelda SM, Wiewrodt R, Zuckerman JB . Gene therapy for lung disease: hype or hope? Ann Intern Med 2000; 132: 649–660.
Chadwick SL et al. Safety of a single aerosol administration of escalating doses of the cationic lipid GL-67/DOPE/DMPE-PEG5000 formulation to the lungs of normal volunteers. Gene Therapy 1997; 4: 937–942.
Bragonzi A et al. Comparison between cationic polymers and lipids in mediating systemic gene delivery to the lungs. Gene Therapy 1999; 6: 1995–2000.
Gautam, Densmore CL, Xu B, Waldrep JC . Enhanced gene expression in mouse lung after PEI–DNA aerosol delivery. Mol Ther 2000; 2: 63–70.
Barron L, Uyechi L, Szoka FC . Cationic lipids are essential for gene delivery mediated by intravenous administration of lipoplexes. Gene Therapy 1999; 6: 1179–1183.
Li S, Huang L . In vivo gene transfer via intravenous administration of cationic lipid–protamine–DNA (LPD) complexes. Gene Therapy 1997; 4: 891–900.
Song YK, Liu F, Chu S, Liu D . Characterization of cationic liposome-mediated gene transfer in vivo by intravenous administration. Hum Gene Ther 1997; 8: 1585–1594.
Tan Y et al. Sequential injection of cationic liposome and plasmid DNA effectively transfects the lung with minimal inflammatory toxicity. Mol Ther 2001; 3: 673–682.
Uyechi LS, Gagne L, Thurston G, Szoka Jr FC . Mechanism of lipoplex gene delivery in mouse lung: binding and internalization of fluorescent lipid and DNA components. Gene Therapy 2001; 8: 828–836.
Yonemitsu Y et al. Efficient gene transfer to airway epithelium using recombinant Sendai virus. Nat Biotechnol 2000; 18: 970–973.
Stern M et al. The effects of jet nebulisation on cationic liposome-mediated gene transfer in vitro. Gene Therapy 1998; 5: 583–593.
Weiss D . Delivery of gene transfer vectors to lung: obstacles and the role of adjunct techniques for airway administration. Mol Therapy 2002; 6: 148.
Somiari S et al. Theory and in vivo application of electroporative gene delivery. Mol Therapy 2000; 2: 178–187.
Ausubel FM et al. Current Protocols in Molecular Biology. John Wiley & Sons: New York, 1994.
Mathiesen I . Electropermeabilization of skeletal muscle enhances gene transfer in vivo. Gene Therapy 1999; 6: 508–514.
Mir LM et al. High-efficiency gene transfer into skeletal muscle mediated by electric pulses. Proc Natl Acad Sci USA 1999; 96: 4262–4267.
Aihara H, Miyazaki J . Gene transfer into muscle by electroporation in vivo. Nat Biotechnol 1998; 16: 867–870.
Heller R et al. In vivo gene electroinjection and expression in rat liver. FEBS Lett 1996; 389: 225–228.
Suzuki T et al. Direct gene transfer into rat liver cells by in vivo electroporation. FEBS Lett 1998; 425: 436–440.
Harrison RL, Byrne BJ, Tung L . Electroporation-mediated gene transfer in cardiac tissue. FEBS Lett 1998; 435: 1–5.
Dujardin N et al. In vivo assessment of skin electroporation using square wave pulses. J Control Release 2002; 79: 219–227.
Martin JB, Young JL, Benoit JN, Dean DA . Gene transfer to intact mesenteric arteries by electroporation. J Vasc Res 2000; 37: 372–380.
Matsumoto T et al. Successful and optimized in vivo gene transfer to rabbit carotid artery mediated by electronic pulse. Gene Therapy 2001; 8: 1174–1179.
Oshima Y et al. Targeted gene transfer to corneal endothelium in vivo by electric pulse. Gene Therapy 1998; 5: 1347–1354.
Blair-Parks K, Weston BC, Dean DA . Gene delivery to the cornea by plasmid injection and electroporation. J Gene Med 2002; 4: 92–100.
Tsujie M et al. Electroporation-mediated gene transfer that targets glomeruli. J Am Soc Nephrol 2001; 12: 949–954.
Dean BS, Byrd Jr JN, Dean DA . Nuclear targeting of plasmid DNA in human corneal cells. Cur Eye Res 1999; 19: 66–75.
Vacik J, Dean BS, Zimmer WE, Dean DA . Cell-specific nuclear import of plasmid DNA. Gene Therapy 1999; 6: 1006–1014.
Wheeler CJ et al. A novel cationic lipid greatly enhances plasmid DNA delivery and expression in mouse lung. Proc Natl Acad Sci USA 1996; 93: 11454–11459.
Stern M et al. Pretreatment with cationic lipid-mediated transfer of the Na+ K+-ATPase pump in a mouse model in vivo augments resolution of high permeability pulmonary oedema. Gene Therapy 2001; 7: 960–966.
Li S et al. Muscle-specific enhancement of gene expression by incorporation of the SV40 enhancer in the expression plasmid. Gene Therapy 2001; 8: 494–497.
Gabriel C, Lau RW, Gabriel S . The dielectric properties of biological tissues: III. Parametric models for the dielectric spectrum of tissues. Phys Med Biol 1996; 41: 2271–2293.
Yew NS et al. Optimization of plasmid vectors for high-level expression in lung epithelial cells. Hum Gene Ther 1997; 8: 575–584.
Yew NS et al. High and sustained transgene expression in vivo from plasmid vectors containing a hybrid ubiquitin promoter. Mol Therapy 2001; 4: 75–82.
Canonico AE, Conary JT, Meyrick BO, Brigham KL . Aerosol and intravenous transfection of human alpha 1-antitrypsin gene to lungs of rabbits. Am J Respir Cell Mol Biol 1994; 10: 24–29.
Reynolds PN et al. A targetable, injectable adenoviral vector for selective gene delivery to pulmonary endothelium in vivo. Mol Therapy 2000; 2: 562–578.
Dumasius V et al. b2-Adrenergic receptor overexpression increases alveolar fluid clearance and responsiveness to endogenous catecholamines in rats. Circ Res 2001; 89: 907–914.
Weiss DJ, Bonneau L, Liggitt D . Use of perfluorochemical liquid allows earlier detection of gene expression and use of less vector in normal lung and enhances gene expression in acutely injured lung. Mol Therapy 2001; 3: 734–745.
Goula D et al. Rapid crossing of the pulmonary endothelial barrier by polyethylenimine/DNA complexes. Gene Therapy 2000; 7: 499–504.
Cassidy KJ et al. A rat lung model of instilled liquid transport in the pulmonary airways. J Appl Physiol 2001; 90: 1955–1967.
Acknowledgements
We thank Joseph N Benoit (University of North Dakota), and G.R. Scott Budinger, Joshua Meeks, and Chris Capaccio (Northwestern University) for insightful discussions. This work was supported in part by grants from the Crane Asthma Fund and the Sandler Program for Asthma Research.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dean, D., Machado-Aranda, D., Blair-Parks, K. et al. Electroporation as a method for high-level nonviral gene transfer to the lung. Gene Ther 10, 1608–1615 (2003). https://doi.org/10.1038/sj.gt.3302053
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.gt.3302053
Keywords
This article is cited by
-
Gene transfer of MRCKα rescues lipopolysaccharide-induced acute lung injury by restoring alveolar capillary barrier function
Scientific Reports (2021)
-
Caveolin-1 gene therapy inhibits inflammasome activation to protect from bleomycin-induced pulmonary fibrosis
Scientific Reports (2019)
-
Electroporation-mediated delivery of FER gene enhances innate immune response and improves survival in a murine model of pneumonia
Gene Therapy (2018)
-
β1-Na+,K+-ATPase gene therapy upregulates tight junctions to rescue lipopolysaccharide-induced acute lung injury
Gene Therapy (2016)
-
Electroporation-mediated delivery of the FER gene in the resolution of trauma-related fatal pneumonia
Gene Therapy (2016)