Abstract
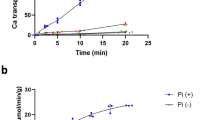
CATION pumps bind and translocate ions with the intermediate formation of a phosphoenzyme. In spite of extensive knowledge of the primary and even secondary structures of several of these cation transport enzymes, however, no high affinity cation binding sites have yet been determined. Here we report the use of oligo-nucleotide-directed, site-specific mutagenesis to identify the amino acids involved in Ca2+ binding in one of these transport enzymes, the Ca2+-ATPase of sarcoplasmic reticulum. Alteration of Glu 309, Glu 771, Asn 796, Thr 799, Asp 800 or Glu 908, each of which is predicted to lie near the centre of the transmembrane domain in putative transmembrane sequences M4, M5, M6 and M81–3 resulted in complete loss of Ca2+ transport function and of Ca2+-dependent phosphorylation of the enzyme by ATP. Phosphorylation of each of the mutant enzymes with inorganic phosphate was observed, however, even in the presence of Ca2+, which inhibits phosphorylation in the wild-type enzyme possessing an intact high affinity Ca2+-binding site. These results suggest that at least six polar, oxygen-containing residues lying near the centre of the transmembrane domain provide ligands for one or both of the two high affinity Ca2+ binding sites in the Ca2+-ATPase.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
MacLennan, D. H., Brandl, C. J., Korczak, B. & Green, N. M. Nature 316, 696–700 (1985).
Brandl, C. J., Green, N. M., Korczak, B. & MacLennan, D. H. Cell 44, 595–607 (1986).
Clarke, D. M. et al. J. biol. Chem. 264 (in the press).
Inesi, G. A. Rev. Physiol. 47, 573–601 (1985).
Inesi, G. J. biol. Chem. 262, 16338–16342 (1987).
Petithory, J. R. & Jencks, W. P. Biochemistry 27, 5553–5564 (1988).
MacLennan, D. H. & Reithmeier, R. A. F. in Structure and Function of Sarcoplasmic Reticulum (eds S. Fleischer and Y. Tonomura) pp. 91–100 (Academic, London, 1985).
Maruyama, K. & MacLennan, D. H. Proc. natn. Acad. Sci. U.S.A. 85, 3314–3318 (1988).
Lytton, J. & MacLennan, D. H. J. biol. Chem. 263, 15024–15031 (1988).
Williams, R. J. P. CIBA Fdn Symp. 122, 145–159 (1986).
Masuda, H. & de Meis, L. Biochemistry 12, 4581–4585 (1973).
de Meis, L. & Masuda, H. Biochemistry 13, 2057–2062 (1974).
Tanford, C., Reynolds, J. A. & Johnson, E. A. Proc. natn. Acad. Sci. U.S.A. 84, 7094–7098 (1987).
Shull, G. E. & Greeb, J. J. biol. Chem. 263, 8646–8657 (1988).
Shull, G. E., Schwartz, A. & Lingrel, J. B. Nature 316, 691–695 (1985).
Shull, G. E. & Lingrel, J. B. J. biol. Chem. 261, 16788–16791 (1986).
Serrano, R., Kielland-Brandt, M. C. & Fink, G. R. Nature 319, 689–693 (1986).
Boyer, P. D. Trends Biochem. Sci. 13, 5–7 (1988).
Kunkel, T. A. Proc. natn. Acad. Sci. U.S.A. 82, 488–492 (1985).
Wong, G. G. et al. Science 228, 810–815 (1985).
Zubrzycka-Gaarn, E., MacDonald, G., Phillips, L., Jorgensen, A. O. & MacLennan, D. H. J. Bioenerg. Biomembr. 16, 442–464 (1984).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Clarke, D., Loo, T., Inesi, G. et al. Location of high affinity Ca2 +-binding sites within the predicted transmembrahe domain of the sarco-plasmic reticulum Ca2+-ATPase. Nature 339, 476–478 (1989). https://doi.org/10.1038/339476a0
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1038/339476a0
This article is cited by
-
Crystal structure of a Na+-bound Na+,K+-ATPase preceding the E1P state
Nature (2013)
-
SERCA mutant E309Q binds two Ca2+ions but adopts a catalytically incompetent conformation
The EMBO Journal (2013)
-
The second sodium pump: from the function to the gene
Pflügers Archiv - European Journal of Physiology (2012)
-
A structural overview of the plasma membrane Na+,K+-ATPase and H+-ATPase ion pumps
Nature Reviews Molecular Cell Biology (2011)
-
Calcium and copper transport ATPases: analogies and diversities in transduction and signaling mechanisms
Journal of Cell Communication and Signaling (2011)