Abstract
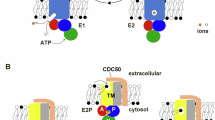
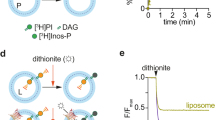
THE Ca2+-dependent binding of annexin proteins to secretory granule membranes seems to be involved in the early stage of exocytosis1—3. Binding studies have shown that these proteins have a specificity for phosphatidylserine (PtdS) interfaces4,5. Further-more, aminolipids are necessary for contact and fusion between lipid vesicles6 or between liposomes and chromaffin granules7. Thus, PtdS must be present on the granule outer (cytoplasmic) monolayer. We report here that chromaffin granules possess a mechanism to maintain PtdS orientation, comparable to the ATP-dependent aminophospholipid translocase from human erythrocytes8-14. The translocase, in granules, selectively transports PtdS from the luminal to the cytoplasmic monolayer, provided the incubation medium contains ATP. As this protein shares several properties with the granule vanadate-sensitive ATPase II15,16, we infer that this ATPase, of relative molecular mass 115,000, is the protein responsible for aminophospholipid translocation. This is the first evidence for an ATP-dependent specific phospholipid 'flippase' in intracellular organelles.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Sudhof, T. C., Walker, J. H. & Obrocki, J. EMBO J. 1, 1167–1170 (1982).
Drust, D. S. & Creutz, C. E. Nature 331, 88–91 (1988).
Odenwald, W. F. & Morris, S. J. Biochem. biophys. Res. Commun. 112, 147–154 (1983).
Geisow, M. J. & Walker, J. H. Trends biochem. Sci. 11, 420–423 (1986).
Klee, C. Biochemistry 27, 6645–6653 (1988).
Papahadjopoulos, D., Vail, W. J., Pangborn, W. A. & Poste, G. Biochim. biophys. Acta 448, 265–283 (1976).
Nayar, R., Hope, M. J. & Cullis, P. R. Biochemistry 21, 4583–4589 (1982).
Seigneuret, M. & Devaux, P. F. Proc. natn. Acad. Sci. U.S.A. 81, 3751–3755 (1984).
Daleke, D. L. & Huestis, W. H. Biochemistry 24, 5406–5416 (1985).
Zachowski, A., Favre, E., Cribier, S., Hervé, P. & Devaux, P. F. Biochemistry 25, 2585–2590 (1986).
Tilley, L., Cribier, S., Roelofsen, B., Op den Kamp, J. A. F. & van Deenen, L. L. M. FEBS Lett. 194, 21–27 (1986).
Connor, J. & Schroit A. J. Biochemistry 27, 848–851 (1988).
Devaux, P. F. FEBS Lett. 234, 8–12 (1988).
Morrot, G., Hervé, P., Zachowski, A., Fellmann, P. & Devaux, P. F. Biochemistry 28, 3456–3462 (1989).
Apps, D. K. & Percy, J. M. Ann. N.Y. Acad. Sci. 493, 178–187 (1987).
Moriyama, Y. & Nelson. N. J. biol. Chem. 263, 8521–8527 (1988).
Bretscher, M. Nature 236, 11–12 (1972).
Op den Kamp, J. A. F. A. Rev. Biochem. 48, 47–71 (1979).
Sune, A., Bette-Bobillo, P., Bienvenue, A., Fellmann, P. & Devaux, P. F. Biochemistry 26, 2972–2978 (1987).
Sune, A., Vidal, M., Morin, P., Sainte-Marie, J. & Bienvenue, A. Biochim. biophys. Acta 946, 315–327 (1988).
Martin, O. C. & Pagano, R. E. J. biol. Chem. 262, 5890–5898 (1987).
Zachowski, A., Fellmann, P. & Devaux, P. F. Biochim. biophys. Acta 815, 510–514 (1985).
Sarkadi, B., Szasz, I. & Gardos, G. Biochim. biophys. Acta 598, 326–338 (1980).
Scherman, D., Soumarmon, A. & Henry, J. P. Biochimie 68, 1303–1309 (1986).
Knight, D. E. & Baker, P. F. J. Membrane Biol. 68, 107–140 (1982).
Diebler, M. F. & Lazereg, S. J. Neurochem. 44, 1633–1641 (1985).
Xie, X. S., Stone, D. K. & Racker, E. J. biol. Chem. 259, 11676–11678 (1984).
Bishop, W. R. & Bell, R. M. Cell 42, 51–60 (1985).
Carty, S. E., Johnson, R. G. & Scarpa, A. Analyt. Biochem. 106, 438–445 (1980).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zachowski, A., Henry, JP. & Devaux, P. Control of transmembrane lipid asymmetry in chromaffin granules by an ATP-dependent protein. Nature 340, 75–76 (1989). https://doi.org/10.1038/340075a0
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1038/340075a0
This article is cited by
-
Exposure of phosphatidylserine on the cell surface
Cell Death & Differentiation (2016)
-
Outside of the box: recent news about phospholipid translocation by P4 ATPases
Journal of Chemical Biology (2012)
-
Phosphatidylserine, a death knell
Cell Death & Differentiation (2001)