Abstract
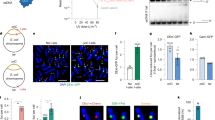
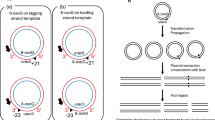
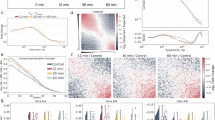
NUCLEOTIDE excision repair helps to ameliorate the lethal and mutagenic consequences of DNA damage by removing helix-distorting lesions from cellular genomes1. We have pre-viously analysed the removal of ultraviolet-induced cyclobutane pyrimidine dimers from specific DNA sequences in mammalian cells and demonstrated that transcriptionally active genes are preferentially repaired2–4. Additionally, we found that in rodent and human cells only the transcribed strand of the dihydrofolate reductase gene is selectively repaired5. Transcription is blocked by pyrimidine dimers in template DNA6 and the selective removal of these lesions seems to be important for cell survival after irradiation with ultraviolet light2,7,8. To determine whether this feature of repair is common to prokaryotes and eukaryotes and better to understand its mechanism, we have investigated repair in the two separate DNA strands of the lactose operon of ultraviolet-irradiated Escherichia coli. We find a dramatic difference in the repair of the two strands only when transcription is induced. Most dimers are removed from the transcribed strand of the induced operon within five minutes of irradiation. In the non-transcribed strand, repair is significantly slower and resembles that found in both strands of the uninduced operon. Thus there seems to be a mechanism that couples nucleotide excision repair and transcription.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Friedberg, E. C. in DNA Repair (Freeman, San Francisco, 1985).
Bohr, V. A., Smith, C. A., Okumoto, D. S. & Hanawalt, P. C. Cell 40, 359–369 (1985).
Madhani, H. D., Bohr, V. A. & Hanawalt, P. C. Cell 45, 417–423 (1986).
Mellon, I., Bohr, V. A., Smith, C. A. & Hanawalt, P. C. Proc. natn. Acad. Sci. U.S.A. 83, 8878–8882 (1986).
Mellon, I., Spivak, G. & Hanawalt, P. C. Cell 51, 241–249 (1988).
Sauerbier, W. & Hercules, K. A. Rev. Genet. 12, 329–363 (1978).
Mayne, L. V. & Lehmann, A. R. Cancer Res. 42, 1493–1498 (1982).
Mayne, L. V., Mullenders, L. H. F. & van Zeeland, A. A. in Mechanisms and Consequences of DNA Damage Processing (eds Friedberg, E. C. & Hanawalt, P. C.) 349–353 (Liss, New York, 1988).
The Lactose Operon (Cold Spring Harbor Laboratory, New York, 1970).
Bohr, V. A. & Okumoto, D. S. in DNA Repair: A Laboratory Manual of Research Procedures (eds Friedberg, E. C. & Hanawalt, P. C.) 3, 347–366 (Dekker, New York, 1988).
Sancar, A. & Rupp, W. D. Cell 33, 249–260 (1983).
Miller, J. H. in Experiments in Molecular Genetics 352–355 (Cold Spring Harbor Laboratory, Cold Spring Harbor, 1972).
Bockrath, R. C. & Palmer, J. E. Molec. gen. Genet 156, 133–140 (1977).
Engstrom, J., Larsen, S., Rogers, S. & Bockrath, R. C. Mutation Res. 132, 143–152 (1984).
Bockrath, R. C., Barlow, A. & Engstrom, J. Mutation Res. 183, 241–247 (1987).
Reed, J. & Hutchinson, F. Molec. gen. Genet. 208, 446–449 (1987).
Vrieling, H. et al. Molec. cell. Biol. 9, 1277–1283 (1989).
Herman, R. K. & Dworkin, N. B. J. Bact. 106, 543–550 (1971).
Savić, D. J. & Kanazir, D. T. Molec. gen. Genet. 118, 45–50 (1972).
Shi, Y. B., Gamper, H. & Hearst, J. E. Nucleic Acids Res. 15, 6843–6854 (1987).
Fornace, A. J., Jr. Alamo, I., Jr, & Hollander, M. C. Proc. natn. Acad. Sci. U.S.A. 85, 8800–8804 (1988).
Hoeijmakers, J. H. J. et al. in Mechanisms and Consequences of DNA Damage Processing (eds Friedberg, E. C. & Hanawalt, P. C.) 281–287 (Liss, New York, 1988).
Thompson, L. H., Weber, C. A. & Carrano, A. V. in Mechanisms and Consequences of DNA Damage Processing (eds Friedberg, E. C. & Hanawalt, P. C.) 289–293 (Liss, New York, 1988).
Sancar, A. & Sancar, G. W. A. Rev. Biochem. 57, 29–67 (1988).
Grossman, L., Caron, P. R., Mazur, S. J. & Oh, E. Y. Fedn Proc. 2, 2696–2701 (1988).
Sancar, A., Franklin, K. A. & Sancar, G. B. Proc. natn. Acad. Sci. U.S.A. 81, 7397–7401 (1984).
Bilofsky, H. S. et al. Nucleic Acids Res. 14, 1–4 (1986).
Ganesan, A. K. & Spivak, G. in DNA Repair: A Laboratory Manual of Research Procedures (eds Friedberg, E. C. & Hanawalt, P. C.) 3, 295–310 (Dekker, New York, 1988).
Smith, C. A., Cooper, P. K. & Hanawalt, P. C. in DNA Repair: A Laboratory Manual of Research Procedures (eds Friedberg, E. C. & Hanawalt, P. C.) 1B, 289–305 (Dekker, New York, 1981).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mellon, I., Hanawalt, P. Induction of the Escherichia coli lactose operon selectively increases repair of its transcribed DNA strand. Nature 342, 95–98 (1989). https://doi.org/10.1038/342095a0
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1038/342095a0
This article is cited by
-
Pervasive Transcription-coupled DNA repair in E. coli
Nature Communications (2022)
-
Single-molecule live-cell imaging visualizes parallel pathways of prokaryotic nucleotide excision repair
Nature Communications (2020)
-
Single-molecule imaging reveals molecular coupling between transcription and DNA repair machinery in live cells
Nature Communications (2020)
-
Mitochondrial reactive oxygen species cause major oxidative mitochondrial DNA damages and repair pathways
Journal of Biosciences (2020)
-
Genomic instability during reprogramming by nuclear transfer is DNA replication dependent
Nature Cell Biology (2017)