Abstract
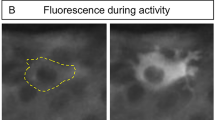
THE increase in intracellular Ca2+concentration ([Ca2+]i) that follows electrical activity in excitable cells influences various cellular functions. But the measured increase in average cytosolic [Ca2+]i(typically 10 nM per action potential1–3) is often less than the micromolar [Ca2+]i required to activate proteins controlling cell function4–5. We now report that clustering of L-type Ca2+ channels causes [Ca2+]i hotspots of average diameter 7 μm at the neuronal growth cone. At the hotspot, [Ca2+]i changes of the order of 1 μM were recorded during 1-s voltage-clamp depolarizations, whereas a single action potential raised [Ca2+]i by 89 nM. Depolarization will therefore activate enzymes with a micromolar requirement for Ca2+ at the hotspot. Local morphological changes near the site of the hotspot were induced by action potentials. We observed hotspots in all regions of the growth cone, usually at the base of processes extending from the growth-cone palm, but never in the neurite. The role of voltage-dependent Ca2+ influx in controlling nerve cell outgrowth has been a puzzle: although raised [Ca2+]i triggers outgrowth of the growth cone margin, neurite elongation requires low [Ca2+]i(refs 6–8). Our results resolve this paradox: electrical activity can selectively raise [Ca2+]i in the growth cone, leaving neurite [Ca2+]ilow. Gross [Ca2+]i gradients and localized hotspots have been previously reported in depolarized neurons3,9–12. Patch-clamp, toxin-binding and freeze-fracture studies have demonstrated that calcium channels are grouped in clusters13–17. However, this is the first report that calcium channel clusters can cause [Ca2+]i hotspots, and that channel clustering has a physiological role.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Smith, S. J., MacDermott, A. B. & Weight, F. F. Nature 304, 350–352 (1983).
Neering, I. R. & McBurney, R. N. Nature 309, 158–160 (1984).
Bolsover, S. R. & Spector, I. J. Neurosci. 6, 1934–1940 (1986).
Baker, P. F. & Knight, D. E. Phil. Trans. Roy. Soc. B296, 83–103 (1981).
Manalan, A. S. & Klee, C. B. Adv. Cyclic Nucleotide 18, 227–278 (1984).
Mattson, M. P. & Kater, S. B. J. Neurosci. 7, 4034–4043 (1987).
Goldberg, D. J. J. Neurosci. 8, 2596–2605 (1988).
Silver, R. A., Lamb, A. G. & Bolsover, S. R. J. Neurosci. 9, 4007–4020 (1989).
Kater, S. B., Mattson, M. P., Cohan, C. & Connor, J. Trends Nueurosci. 11, 315–321 (1988).
Smith, S. J. & Augustine, G. J. Trends Neurosci. 11, 458 (1988).
Tank, D. W., Sugimori, M., Connor, J. A. & Llinas, R. R. Science 242, 773–777 (1988).
Regehr, W. G., Connor, J. A. & Tank, D. W. Nature 341, 533–536 (1989).
Lipscombe, D. et al. Proc. natn. Acad. Sci. U.S.A. 85, 2398–2402 (1988).
Thompson, S. & Coombs, J. J. Neurosci. 8, 1929–1939 (1988).
Fox, A. P., Nowycky, M. C. & Tsien, R. W. J. Physiol. 394, 173–200 (1987).
Jones, O. T., Kunze, D. L. & Angelides, K. J. Science 244, 1189–1193 (1989).
Pumplin, D. W., Reese, T. S. & Linas, R. Proc. natn. Acad. Sci. U.S.A. 78, 7210–7213 (1981).
Bolsover, S. R. J. gen. Physiol. 88, 149–165 (1986).
Narahashi, T., Tsunoo, A. & Yoshii, M. J. Physiol. 383, 231–249 (1987).
Tsien, R. W., Lipscombe, D., Madison, D. V., Bley, K. R. & Fox, A. P. Trends Neurosci. 11, 431–438 (1988).
Nowycky, M. C., Fox, A. P. & Tsien, R. W. Nature 316, 440–443 (1985).
O'Sullivan, A. J., Cheek, T. R., Moreton, R. B., Berridge, M. J. & Burgoyne, R. D. EMBO J. 8, 401–411 (1989).
Cooper, J. A. et al. J. Cell Biol. 104, 491–501 (1987).
Purves, D., Lichtman, J. W. Principles of Neural Development (Sinauer, Sunderland, Massachusetts, 1985).
Gundersen, R. W. & Barrett, J. M. J. Cell Biol. 87, 546–554 (1980).
Grynkiewicz, G., Poenie, M. & Tsien, R. Y. J. biol. Chem. 260, 3440–3540 (1975).
Lansman, J. B., Hess, P. & Tsien, R. W. J. gen. Physiol. 88, 321–347 (1986).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Silver, R., Lamb, A. & Bolsover, S. Calcium hotspots caused by L-channel clustering promote morphological changes in neuronal growth cones. Nature 343, 751–754 (1990). https://doi.org/10.1038/343751a0
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1038/343751a0
This article is cited by
-
The molecular basis for calcium-dependent axon pathfinding
Nature Reviews Neuroscience (2006)
-
Cytolocalization of ion-channel antagonist binding sites in sunflower protoplasts during the early steps of culture
Protoplasma (1999)
-
Activity-dependent changes in voltage-dependent calcium currents and transmitter release
Molecular Neurobiology (1997)