Abstract
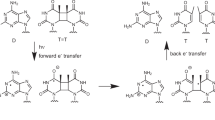
CYCLOBUTANE and [6–4]-pyrimidine dimers are major photo-products of ultraviolet-irradiated DNA. The yield of these photo-products is dependent on the sequence1,2 and structure3 of the DNA. By analysing the photofootprints of fragments produced by cleavage of the DNA chain near [6–4]-pyrimidine dimers4, we show here that a homopurine-homopyrimidine insert (with either d(TC)x or d(C)n) in plasmid pUC19 is, as expected, a good target for UV-induced pyrimidine-dimer formation. But we find that dimeriz-ation is virtually completely suppressed when the pyrimidine oligonucleotides dfTC)y or d(C) m are added to DNA carrying d(TC)x- or d(C)n-containing inserts, respectively. This effect is dependent on the type of oligonucleotide used and is site-specific. The protection occurs under acidic conditions that favour the formation of intermolecular triplexes between the homopurine-homopyrimidine inserts and homologous oligopyrimidines5. We therefore conclude that triplex formation effectively protects the DNA duplex from UV-induced damage (pyrimidine dimerization). This observation makes the photofootprinting assay6 a very promising method for studying intermolecular and intramolecular triplexes (?-form DNA) both in vitro and in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Gordon, L.K. & Haseltine, W. A. Radiat. Res. 89, 99 (1982).
Becker, M. M. & Wang, Z. J. molec. Biol. 210, 429–438 (1989).
Becker, M. M. & Wang, Z. J. biol. Chem. 264, 4163–4167 (1989).
Lippke, J. A., Gordon, L. K., Brash, D. E. & Haseltine, W. A. Proc. natn. Acad. Sci. U.S.A. 78, 3388–3392 (1981).
Lyamichev, V. I., Mirkin, S. M., Frank-Kamenetskii, M. D. & Cantor, C. R. Nucleic Acids Res. 16, 2165–2178 (1988).
Becker, M. M. & Wang, J. C. Nature 309, 682–687 (1984).
Lyamichev, V. I., Mirkin, S. M. & Frank-Kamenetskii, M. D. J. biomolec. Struct. Dyn. 5, 275–282 (1987).
Lyamicnev, V. I. et al. Nucleic Acids Res. 17, 9417–9423 (1989).
Friedberg, E. C. DNA Repair (Freeman, New York, 1985).
Arnott, S., Bond, P. J., Seising, E. & Smith, P. J. C. Nucleic Acids Res. 3, 2459–2470 (1976).
Lyamichev, V. I., Mirkin, S. M. & Frank-Kamenetskii, M. D. J. biomolec. Struct. Dyn. 3, 667–669 (1986).
Mirkin, S. M. et al. Nature 330, 495–497 (1987).
Voloshin, O. N. et al. Nature 333, 475–476 (1988).
Lyamichev, V. I., Mirkin, S. M. & Frank-Kamenetskii, M. D. J. biolmolec. Struct. Dyn. 3, 327–338 (1985).
Bernues J., Beltran, R., Casasnovas, J. M. & Azorin, F. EMBO J. 8, 2087–2094 (1989).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lyamichev, V., Frank-Kamenetskii, M. & Soyfer, V. Protection against UV-induced pyrimidine dimerization in DNA by triplex formation. Nature 344, 568–570 (1990). https://doi.org/10.1038/344568a0
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1038/344568a0