Abstract
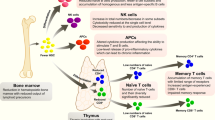
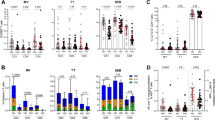
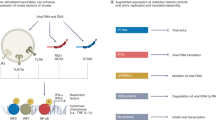
Vaccines against a variety of infectious diseases represent one of the great triumphs of medicine. The immune correlates of protection induced by most current vaccines seem to be mediated by long-lived humoral immune responses. By contrast, there are no currently available vaccines that are uniformly effective for diseases such as HIV, malaria and tuberculosis, in which the cellular immune response might be crucial in mediating protection. Here we examine the mechanisms by which long-lived cellular immune responses are generated and maintained in vivo. We then discuss current approaches for vaccination against diseases in which cellular immune responses are important for protection.
Similar content being viewed by others
Enjoying our latest content?
Log in or create an account to continue
- Access the most recent journalism from Nature's award-winning team
- Explore the latest features & opinion covering groundbreaking research
or
References
Ahmed, R. & Gray, D. Immunological memory and protective immunity: understanding their relation. Science 272 , 54–60 (1996).
Dutton, R. W., Bradley, L. M. & Swain, S. L. T cell memory. Annu. Rev. Immunol. 16, 201–223 (1998).
Sallusto, F., Lenig, D., Forster, R., Lipp, M. & Lanzavecchia, A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401, 708–712 (1999).
Gray, D. & Matzinger, P. T cell memory is short-lived in the absence of antigen. J. Exp. Med. 174, 969–974 (1991).
Bachmann, M. F., Kundig, T. M., Hengartner, H. & Zinkernagel, R. M. Protection against immunopathological consequences of a viral infection by activated but not resting cytotoxic T cells: T cell memory without ‘memory T cells’? Proc. Natl Acad. Sci. USA 94, 640–645 (1997).
Kundig, T. M. et al. On the role of antigen in maintaining cytotoxic T-cell memory . Proc. Natl Acad. Sci. USA 93, 9716– 9723 (1996).
Lau, L. L., Jamieson, B. D., Somasundaram, T. & Ahmed, R. Cytotoxic T-cell memory without antigen. Nature 369 , 648–652 (1994).
Tanchot, C., Lemonnier, F. A., Perarnau, B., Breitas, A. A. & Rocha, B. Differential requirements for survival and proliferation of CD8 naïve or memory T cells. Science 276, 2057–2062 ( 1997).
Murali-Krishna, K. et al. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science 286, 1377– 1381 (1999).
Doolan, D. L. et al. Circumventing genetic restriction of protection against malaria with multigene DNA immunization: CD8+ cell-, interferon γ-, and nitric oxide-dependent immunity. J. Exp. Med. 183 , 1739–1746 (1996).
Guebre-Xabier, M., Schwenk, R. & Krzych, U. Memory phenotype CD8+ T cells persist in livers of mice protected against malaria by immunization with attenuated Plasmodium berghei sporozoites. Eur. J. Immunol. 29, 3978–3986 (1999).
Scheller, L. F. & Azad, A. F. Maintenance of protective immunity against malaria by persistent hepatic parasites derived from irradiated sporozoites. Proc. Natl Acad. Sci. USA 92, 4066–4068 (1995).
Matano, T. et al. Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J. Virol. 72 , 164–169 (1998).
Schmitz, J. E. et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283, 857–860 (1999).
Ogg, G. S. et al. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science 279, 2103 –2106 (1998).
Kalams, S. A. et al. Levels of human immunodeficiency virus type 1-specific cytotoxic T-lymphocyte effector and memory responses decline after suppression of viremia with highly active antiretroviral therapy. J. Virol. 73, 6721–6728 (1999).
Ortiz, G. M. et al. HIV-1-specific immune responses in subjects who temporarily contain virus replication after discontinuation of highly active antiretroviral therapy. J. Clin. Invest. 104, R13– R18 (1999).
Swain, S. L., Hu, H. & Huston, G. Class II-independent generation of CD4 memory T cells from effectors. Science 286, 1381–1383 ( 1999).
Garcia, S., DiSanto, J. & Stockinger, B. Following the development of a CD4 T cell response in vivo: from activation to memory formation. Immunity 11, 163–171 (1999).
Schuback, A. et al. Detection of Leishmania DNA by polymerase chain reaction in scars of treated human patients. J. Infect. Dis. 178, 911–914 (1998).
Zhang, X., Sun, S., Hwang, I., Tough, D. F. & Sprent, J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity 8, 591–599 (1998).
Gurunathan, S., Prussin, C., Sacks, D. L. & Seder, R. A. Vaccine requirements for sustained cellular immunity to an intracellular parasitic infection. Nature Med. 4, 1409– 1415 (1998).
Schneider, J. et al. Enhanced immunogenicity for CD8+ T cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nature Med. 4, 397–402 (1998).
Oehen, S., Waldner, H., Kundig, T. M., Hengartner, H. & Zinkernagel, R. M. Antivirally protective cytotoxic T cell memory to lymphocytic choriomeningitis virus is governed by persisting antigen. J. Exp. Med. 176, 1273– 1281 (1992).
McHeyzer-Williams, M. G. & Davis, M. M. Antigen-specific development of primary and memory T cells in vivo. Science 268, 106–111 ( 1995).
Razvi, E. S. & Welsh, R. M. Apoptosis in viral infections. Adv. Virus Res. 45, 1–60 (1995).
Ochsenbein, A. F. et al. A comparison of T cell memory against the same antigen induced by virus versus intracellular bacteria. Proc. Natl Acad. Sci. USA 96, 9293–9298 ( 1999).
Opferman, J. T., Ober, B. T. & Ashton-Rickardt, P. G. Linear differentiation of cytotoxic effectors into memory T lymphocytes. Science 283, 1745– 1748 (1999).
Mullbacher, A. The long-term maintenance of cytotoxic T cell memory does not require persistence of antigen. J. Exp. Med. 179, 317– 321 (1994).
Hou, S., Hyland, L., Ryan, K. W., Portner, A. & Doherty, P. C. Virus-specific CD8+ T-cell memory determined by clonal burst size. Nature 369, 652– 654 (1994).
Flynn, K. J. et al. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity 8, 683–691 (1998).
Zhang, Z. et al. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science 286, 1353–1357 (1999).
Lifson, J. D. et al. The extent of early viral replication is a critical determinant of the natural history of simian immunodeficiency virus infection. J. Virol. 71, 9508–9514 (1997).
Ulmer, J. B. et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science 259, 1745–1749 (1993).
Seder, R. A. & Gurunathan, S. DNA vaccines — designer vaccines for the 21st century. N. Engl. J. Med. 341 , 277–278 (1999).
Wang, R. et al. Induction of antigen-specific cytotoxic T lymphocytes in humans by a malaria DNA vaccine. Science 282, 476 –480 (1998).
Le, T. P. et al. Safety, tolerability and humoral immune responses after intramuscular administration of a malaria DNA vaccine to healthy adult volunteers. Vaccine 18, 1893–1901 ( 2000).
Calarota, S. et al. Cellular cytotoxic response induced by DNA vaccination in HIV-1-infected patients. Lancet 351, 1320 –1325 (1998).
Cho, H. J. et al. Immunostimulatory DNA-based vaccines induce cytotoxic lymphocyte activity by a T-helper cell-independent mechanism. Nature Biotechnol. 18, 509–514 ( 2000).
Cowdery, J. S., Chace, J. H., Yi, A. K. & Krieg, A. M. Bacterial DNA induces NK cells to produce IFN-γ in vivo and increases the toxicity of lipopolysaccharides. J. Immunol. 156, 4570–4575 (1996).
Klinman, D. M., Yi, A. K., Beaucage, S. L., Conover, J. & Krieg, A. M. CpG motifs present in bacterial DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon. Proc. Natl Acad. Sci. USA 93, 2879–2883 (1996).
Sato, Y. et al. Immunostimulatory DNA sequences necessary for effective intradermal gene immunization. Science 273, 352– 354 (1996).
Krieg, A. M. et al. Sequence motifs in adenoviral DNA block immune activation by stimulatory CpG motifs. Proc. Natl Acad. Sci. USA 95, 12631–12636 (1998).
Schneider, J. et al. Induction of CD8+ T cells using heterologous prime-boost immunisation strategies. Immunol. Rev. 170, 29–38 (1999).
Kent, S. J. et al. Enhanced T-cell immunogenicity and protective efficacy of a human immunodeficiency virus type 1 vaccine regimen consisting of consecutive priming with DNA and boosting with recombinant fowlpox virus. J. Virol. 72, 10180–10188 (1998).
Hanke, T. et al. Effective induction of simian immunodeficiency virus-specific cytotoxic T lymphocytes in macaques by using a multiepitope gene and DNA prime-modified vaccinia virus Ankara boost vaccination regimen. J. Virol. 73, 7524–7532 (1999).
Stoute, J. A. et al. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. N. Engl. J. Med. 336, 86–91 (1997).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Seder, R., Hill, A. Vaccines against intracellular infections requiring cellular immunity. Nature 406, 793–798 (2000). https://doi.org/10.1038/35021239
Published:
Issue date:
DOI: https://doi.org/10.1038/35021239
This article is cited by
-
Pan-genome and reverse vaccinology approaches to design multi-epitope vaccine against Epstein-Barr virus associated with colorectal cancer
Immunologic Research (2023)
-
Boosting Tat DNA vaccine with Tat protein stimulates strong cellular and humoral immune responses in mice
Biotechnology Letters (2020)
-
Enterococcus faecium NCIMB 10415 administration improves the intestinal health and immunity in neonatal piglets infected by enterotoxigenic Escherichia coli K88
Journal of Animal Science and Biotechnology (2019)
-
Protective humoral and CD4+ T cellular immune responses of Staphylococcus aureus vaccine MntC in a murine peritonitis model
Scientific Reports (2018)
-
Growth arrested live-attenuated Leishmania infantum KHARON1 null mutants display cytokinesis defect and protective immunity in mice
Scientific Reports (2018)