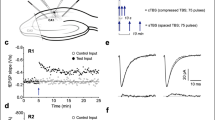
Abstract
LONG-TERM potentiation (LTP) in the hippocampus is thought to contribute to memory formation. In the Ca1 region, LTP requires the NMDA (N-methyl-D-aspartate) receptor-dependent influx of Ca2+ and activation of serine and threonine protein kinases. Because of the high amount of protein tyrosine kinases in hippocampus and cerebellum1,2, two regions implicated in learning and memory, we examined the possible additional requirement of tyrosine kinase activity in LTP. We first examined the specificity in brain of five inhibitors of tyrosine kinase3–5 (Table 1) and found that two of them, lavendustin A and genistein, showed substantially greater specificity for tyrosine kinase from hippocampus6 than for three serine–threonine kinases: protein kinase A, protein kinase C, and Ca2+/calmodulin kinase II. Lavendustin A and genistein selectively blocked the induction of LTP when applied in the bath or injected into the postsynaptic cell. By contrast, the inhibitors had no effect on the established LTP, on normal synaptic transmission, or on the neurotransmitter actions attributable to the actions of protein kinase A or protein kinase C. These data suggest that tyrosine kinase activity could be required postsynaptically for long-term synaptic plasticity in the hippocampus. As Ca2+ cal-modulin kinase II or protein kinase C seem also to be required7, 8, the tyrosine kinases could participate postsynaptically in a kinase network together with serine and threonine kinases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Hirano, A. A. Greengard, P. & Huganir, R. L. J. Neurochem. 50, 1447–1455 (1988).
Pang, D. T., Wang, J. K. T., Valtorta, F., Benefenati, F. & Greengard, P. Proc. natn. Acad. Sci. U.S.A. 85, 762–766 (1988).
Akiyama, T. et al. J. biol. Chem. 31, 14797–14803 (1986).
Onoda, T. et al. J. nat. Prod. 52, 1252–1257 (1989).
Lyall, R. M. et al. J. biol. Chem. 264, 14503–14509 (1989).
Sugrue, M. M., Brugge, J. S., Marshak, D. R., Greengard, P. & Gustafson, E. L. J. Neurosci. 10, 2513–2527 (1990).
Malinow, R., Schulman, H. & Tsien, R. W. Science 245, 862–866 (1989).
Malenka, R. C. et al. Nature 349, 554–557 (1989).
Nicoll, R. A., Malenka, R. C. & Kauer, J. A. Physiol. Rev. 70, 513–565 (1990).
Malenka, R. C., Madison, D. V., Andrade, R. & Nicoll, R. A. J. Neurosci. 6, 475–480 (1986).
Malenka, R. C., Madison, D. V. & Nicoll, R. A. Nature 321, 175–177 (1986).
Madison, D. V. & Nicoll, R. A. Nature 299, 636–638 (1982).
Nicoll, R. A., Kauer, J. A. & Malenka, R. C. Neuron 1, 7–103 (1988).
Migliaccio, A., Rotondi, A. & Auricchio, F. Proc. natn. Acad. Sci. U.S.A. 81, 5921–5925 (1984).
Auricchio, F., Migliaccio, A., Castoria, G., Rotondi, A. & Di Domenico, M. Meth Enzym. 139, 731–743 (1987).
Stratton, K. R., Worley, P. F., Huganir, R. L. & Baraban, J. M. proc. natn. Acad. Sci. U.S.A. 86, 2498–2501 (1989).
Anderson, N. G., Maller, J. L., Tonks, N. K. & Sturgill, T. W. Nature 343, 651–652 (1990).
Bliss, T. V., Douglas, R. M., Errington, M. L. & Lynch, M. A. J. Physiol., Lond. 377, 391–408 (1986).
Bekkers, J. M. & Stevens, C. F. Nature 346, 724–729 (1990).
Malinow, R. & Tsien, R. W. Nature 346, 177–180 (1990).
Bredt, D. S. et al. Nature 351, 714–718 (1991).
Terlau, H. & Seifert, W. Brain Res. 484, 352–356 (1989).
Terlau, H. & Seifert, W. Eur. J. Neurosci. 2, 973–977 (1990).
Walaas, S. I., Lustig, A., Greengard, P. & Brugge, J. S. Molec. Brain Res. 3, 215–222 (1988).
Braun, S., Raymond, W. E. & Racker, E. J. biol. Chem. 259, 2051–2054 (1984).
Kikkawa, U., Takal, Y., Minakuchi, R., Inohgra, S. & Nishizuka, Y. J. biol. Chem. 257, 13341–13348 (1982).
Roskoski, R. Meth. Enzym. 99, 3–7 (1983).
Hashimoto, Y. & Soderling, T. R. Archs Biochem. Biophys. 252, 418–425 (1987).
O'Dell, T. J. et al. Proc. natn. Acad. Sci. U.S.A. (in the press).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
O'Dell, T., Kandel, E. & Grant, S. Long-term potentiation in the hippocampus is blocked by tyrosine kinase inhibitors. Nature 353, 558–560 (1991). https://doi.org/10.1038/353558a0
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1038/353558a0
This article is cited by
-
Diabetic Encephalopathy: Role of Oxidative and Nitrosative Factors in Type 2 Diabetes
Indian Journal of Clinical Biochemistry (2024)
-
Pyk2 in dorsal hippocampus plays a selective role in spatial memory and synaptic plasticity
Scientific Reports (2021)
-
Safflower Yellow Improves Synaptic Plasticity in APP/PS1 Mice by Regulating Microglia Activation Phenotypes and BDNF/TrkB/ERK Signaling Pathway
NeuroMolecular Medicine (2020)
-
Genistein: is the multifarious botanical a natural anthelmintic too?
Journal of Parasitic Diseases (2018)
-
Pyk2 modulates hippocampal excitatory synapses and contributes to cognitive deficits in a Huntington’s disease model
Nature Communications (2017)