Abstract
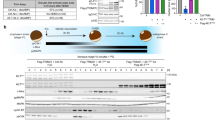
WHEN fully grown Xenopus oocytes are stimulated by progesterone, a period of protein synthesis is necessary for maturation1. Synthesis of the mos proto-oncogene product, pp39mos is necessary for the activation of M-phase promoting factor (MPF) in meiosis I (ref. 2). On the basis that mos is translated de novo on hormonal stimulation of Xenopus oocytes3 and that injecting mos RNA into oocytes induces their maturation3,4, we have proposed that the mos protein is a candidate initiator of oocyte maturation, needed to trigger the conversion of precursor MPF into its active form1,3. To determine whether mos is the only protein required for initiating maturation, we have produced a soluble, active recombinant mos protein and injected it into Xenopus oocytes. We report here that in the absence of protein synthesis that mos protein efficiently induces germinal vesicle breakdown and the activation of MPF. The oocytes, however, do not proceed into meiosis II. Thus, the mos protein fulfills the requirements of an initiator protein, but the synthesis of one or more additional proteins may be necessary to complete oocyte maturation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Wasserman, W. J. & Masui, Y. Expl. Cell Res. 91, 381–388 (1975).
Sagata, N. et al. Nature 335, 519–525 (1988).
Sagata, N. et al. Science 245, 643–646 (1989).
Freeman, R. S. et al. Proc. natn. Acad Sci. U.S.A. 86, 5805–5809 (1989).
Maina, C. V. et al. Gene 74, 365–373 (1988).
Singh, B., Hannink, M., Donoghue, D. J. & Arlinghaus, R. B. J. Virol. 60, 1148–1152 (1986).
Kanki, J. P. & Donoghue, D. J. Proc. natn. Acad. Sci. U.S.A. 88, 5794–5798 (1991).
Daar, I., Paules, R. S. & Vande Woude, G. F. J. Cell Biol. 114, 329–335 (1991).
Sagata, N., Watanabe, N., Vande Woude, G. F. & Ikawa, Y. Nature 342, 512–518 (1989).
Masui, Y. & Markert, C. L. J. exp. Zool. 117, 129–146 (1971).
Gerhart, J., Wu, M. & Kirschner, M. J. Cell Biol. 98, 1247–1255 (1984).
Yew, N., Oskarsson, M., Daar, I., Blair, D. G. & Vande Woude, G. F. Molec. cell. Biol. 11, 604–610 (1991).
Watanabe, N., Vande Woude, G. F., Ikawa, Y. & Sagata, N. Nature 342, 505–511 (1989).
Smith, L. D. Development 107, 685–699 (1989).
Arion, D., Meijer, L., Brizuela, L. & Beach, D. Cell 55, 371–378 (1988).
Minshull, J., Blow, J. J. & Hunt, T. Cell 56, 947–956 (1989).
Murray, A. W. & Kirschner, M. W. Nature 339, 275–280 (1989).
Gautier, J. & Maller, J. L. EMBO J. 10, 177–182 (1991).
Kobayashi, H. et al. J. Cell Biol. 114, 755–765 (1991).
Minshull, J., Murray, A., Colman, A. & Hunt, T. J. Cell Biol. 114, 767–772 (1991).
Wasserman, W. J., Richter, J. D. & Smith, L. D. Devl Biol. 89, 152–158 (1982).
Wallace, R. A. & Misulovin, Z. Proc. natn. Acad. Sci. U.S.A. 75, 5534–5538 (1978).
Felix, M.-A., Pines, J., Hunt, T. & Karsenti, E. EMBO J. 8, 3059–3069 (1989).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yew, N., Mellini, M., Martinez, C. et al. Meiotic initiation by the mos protein in Xenopus. Nature 355, 649–652 (1992). https://doi.org/10.1038/355649a0
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1038/355649a0
This article is cited by
-
Transcriptome dynamics and molecular cross-talk between bovine oocyte and its companion cumulus cells
BMC Genomics (2011)
-
The Xenopus Cell Cycle: An Overview
Molecular Biotechnology (2008)
-
B-Raf and C-Raf are required for Ras-stimulated p42 MAP kinase activation in Xenopus egg extracts
Oncogene (2006)
-
A positive-feedback-based bistable ‘memory module’ that governs a cell fate decision
Nature (2003)
-
Competence to replicate in the unfertilized egg is conferred by Cdc6 during meiotic maturation
Nature (2002)