Abstract
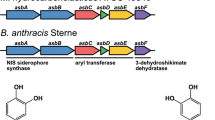
VIRTUALLY all microorganisms require iron for growth. The paucity of iron in surface ocean water (∼0.02−1.0 nM (refs 1, 2)) has spurred a lively debate concerning iron limitation of primary productivity3–6, yet little is known about the molecular mechanisms used by marine microorganisms to sequester iron. Terrestrial bacteria use a siderophore-mediated ferric uptake system7. A sidero-phore is a low-molecular-mass compound with a high affinity for ferric ion which is secreted by microorganisms in response to low-iron environments; siderophore biosynthesis is regulated by iron levels, with repression by high iron. Although open-ocean marine microorganisms (such as phytoplankton8 and bacteria9) produce siderophores, the nature of these siderophores has not been investigated. We report here the first structure determination, to our knowledge, of the siderophores from an open-ocean bacterium, alterobactin A and B from Alteromonas luteoviolacea. A. luteoviol-acea is found in oligotrophic10 and coastal11 waters. Alterobactin A has an exceptionally high affinity constant for ferric ion. We suggest that at least some marine microorganisms may have developed higher-affinity iron chelators as part of an efficient iron-uptake mechanism which is more effective than that of their terrestrial counterparts.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Bruland, K. W., Donat, J. R. & Hutchins, D. A. Limnol. Oceanogr. 36, 1555–1577 (1991).
Martin, J. H., Gordon, R. M. & Fitzwater, S. E. Limnol. Oceanogr. 36, 1793–1802 (1991).
Martin, J. H. & Fitzwater, S. E. Nature 331, 341–343 (1988).
Price, N. M., Andersen, L. F. & Morel, F. M. M. Deep Sea Res. 38, 1361–1378 (1991).
Martin, J. H., Gordon, R. M. & Fitzwater, S. E. Nature 345, 156–158 (1990).
Sunda, W. G., Swift, D. G. & Huntsman, S. A. Nature 351, 55–57 (1991).
Neilands, J. B. Adv. Inorg. Biochem. 8, 63–90 (1990).
Trick, C. G., Andersen, R. J., Gillam, A. & Harrison, P. J. Science 219, 306–308 (1983).
Trick, C. G. Curr. Microbiol. 18, 375–378 (1989).
Andersen, R. J., Wolfe, M. S. & Faulkner, D. J. Mar. Biol. 27, 281–285 (1974).
Gauthier, M. J. & Flatau, G. N. Can. J. Microbiol. 22, 1612–1619 (1976).
Reid, R. T. & Butler, A. Limnol. Oceanogr. 36, 1783–1792 (1991).
Bax, A. & Summers, M. F. J. Am. chem. Soc. 108, 2093–2094 (1986).
Bronxli, P. & Gerig, J. T. J. Am. chem. Soc. 112, 3719–3726 (1990).
Teintze, M. & Leong, J. Biochemistry 20, 6457–6462 (1981).
Salituro, F. G., Agarwal, N., Hofman, T. & Rich, D. H. J. med. Chem. 30, 286–295 (1987).
Mayor, S. & Hosur, R. V. Magn. Res. Chem. 23, 470–473 (1985).
Chaberek, S. & Martell, A. E. Organic Sequestering Agents (Wiley, New York, 1959).
Harris, D. C. Quantitative Chemical Analysis (Freeman, San Francisco, 1982).
Loomis, L. D. & Raymond, K. N. Inorg. Chem. 30, 906–911 (1991).
Perrin, D. D. & Dempsey, B. Buffers for pH and Metal Ion Control (Chapman and Hall, London, 1974).
Jalal, M. A. F. et al. J. Am. chem. Soc. 111, 292–296 (1989).
Takahashi, A. et al. J. Antibiotics 40, 1671–1676 (1987).
Marfey, P. Carlsberg Res. Commun. 49, 591–596 (1984).
Rich, D. H., Sun, E. T. O. & Ulm, E. J. med. Chem. 23, 27–33 (1980).
Schwarzenbach, G. & Schwarzenbach, K. Helv. chim. acta 46, 1390–1400 (1963).
Abdallah, M. A. et al. Abstracts XXVII Int. Conf. Coordination Chemistry, W20 (1989).
Wong, G. B., Kappel, M. J., Raymond, K. N., Matzanke, B. & Winkelmann, G. J. Am. chem. Soc. 105, 810–815 (1983).
Anderegg, G., L'Eplattenier, F. & Schwarzenbach, G. Helv. chim. acta 46, 1409–1422 (1963).
Harris, W. R., Carrano, C. J. & Raymond, K. N. J. Am. chem Soc. 101, 2722–2727 (1979).
Harris, W. R., Carrano, C. J. & Raymond, K. N. J. Am. chem. Soc. 101, 2213–2214 (1979).
Martell, A. E. & Smith, R. M. Critical Stability Constants Vol. 1 (Plenum, New York, 1974).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Reid, R., Livet, D., Faulkner, D. et al. A siderophore from a marine bacterium with an exceptional ferric ion affinity constant. Nature 366, 455–458 (1993). https://doi.org/10.1038/366455a0
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1038/366455a0
This article is cited by
-
Assessing the contribution of diazotrophs to microbial Fe uptake using a group specific approach in the Western Tropical South Pacific Ocean
ISME Communications (2022)
-
The Combined Efficiency of Dietary Isomaltooligosaccharides and Bacillus spp. on the Growth, Hemato-Serological, and Intestinal Microbiota Indices of Caspian Brown Trout (Salmo trutta caspius Kessler, 1877)
Probiotics and Antimicrobial Proteins (2019)
-
β-Hydroxyaspartic acid in siderophores: biosynthesis and reactivity
JBIC Journal of Biological Inorganic Chemistry (2018)
-
Edible Antimicrobial Coating Incorporating a Polymeric Iron Chelator and Its Application in the Preservation of Surimi Product
Food and Bioprocess Technology (2016)
-
Metallophores and Trace Metal Biogeochemistry
Aquatic Geochemistry (2015)