Abstract
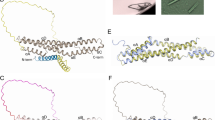
The 2.3-Â crystal structure of the transcription factor NF-κB p50 homodimer bound to a palindromic κB site reveals that the Rel homology region folds into two distinct domains, similar to those in the immunoglobulin superfamily. The p50 dimer envelopes an undistorted B-DNA helix, making specific contacts along the 10-base-pair κB recognition site mainly through loops connecting secondary structure elements in both domains. The carboxy-terminal domains form a dimerization interface between β-sheets using residues that are strongly conserved in the Rel family.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Sen, R. & Baltimore, D. Cell 47, 921–928 (1986).
Blank, V., Kourilsky, P. & Israel, A. Trends Biochem. Sci. 17, 135–140 (1990).
Baeuerle, P. & Henkel, T. A. Rev. Immun. 12, 141–179 (1994).
Grilli, M., Chiu, J.-S. & Lenardo, M. Int. Rev. Cytol. 143, 1–62 (1993).
Liou, H.-C. & Baltimore, D. Curr. Opin. Cell Biol. 5, 477–487 (1993).
Kopp, E. & Ghosh, S. Adv. Immun. 58, 1–27 (1994).
Ghosh, S. et al. Cell 62, 1019–1029 (1990).
Kieran, M. et al. Cell 62, 1007–1018 (1990).
Nolan, G., Ghosh, S., Liou, H.-C., Tempst, P. & Baltimore, D. Cell 64, 961–969 (1991).
Ruben, S. et al. Science 251, 1490–1493 (1991).
Schmid, R. M., Parkins, N. D., Duckett, C. S. & Nabel, G. J. Nature 352, 733–736 (1992).
Ryseck, R.-P. Molec. cell. Biol. 12, 674–684 (1992).
Tony, Y. et al. Cell 75, 753–763 (1993).
Baeuerle, P. A. & Baltimore, D. Science 242, 541–546 (1988).
Zabel, U. & Baeuerle, P. Cell 61, 255–256 (1990).
Palombella, V. J., Rando, O., Goldberg, A. & Maniatis, T. Cell 78, 773–785 (1994).
Ghosh, S. & Baltimore, D. Nature 344, 678–682 (1990).
Luthy, R., Bowie, J. & Einsenberg, D. Nature 356, 83–85 (1992).
Pabo, C. O. & Sauer, R. A. Rev. Biochem. 61, 1053–1095 (1992).
Yunje, C., Gorina, S., Jeffrey, P. & Pavletich, N. Science 265, 346–355 (1994).
Kunsch, C., Ruben, S. M. & Rosen, C. A. Molec. cell. Biol. 12, 4412–4421 (1992).
Winkler, F. K. et al. EMBO. J. 12, 1781–1795 (1993).
Bressler, P. et al. J. Virol. 67, 288–293 (1993).
Kumar, S., Rabson, A. & Gelinus, C. Molec. cell. Biol. 12, 3094–3106 (1992).
Liu, J., Fan, Q. R., Sodeoka, M., Lane, W. S. & Verdine, G. L. Curr. Biol. 1, 47–55 (1994).
Liu, J., Sodeoka, M., Lane, W. S. & Verdine, G. L. Proc. natn. Acad. Sci. U.S.A. 91, 908–912 (1994).
Tolenado, M. B., Ghosh, D., Trinh, F. & Leonard, W. J. Molec. cell. Biol. 13, 852–860 (1993).
Brownwell, E., Mittereder, N. & Rice, N. Oncogene 4, 935–942 (1989).
Northrop, J. P. et al. Nature 369, 497–502 (1994).
Kumar, S. & Gelinus, C. Proc. natn. Acad. Sci. U.S.A. 90, 8962–8966 (1993).
Dimitris, T. & Maniatis, T. Cell 71, 777–789 (1992).
Harpaz, Y. & Chothia, C. J. molec. Biol. 238, 528–539 (1994).
Van Roey, P. & Beerman, T. Proc. natn. Acad. Sci. U.S.A. 86, 6587–6591 (1989).
DeVos, A. M., Ultsch, M. & Kossiakoff, A. A. Science 255, 306–312 (1992).
Haltmark, D. Nature 367, 116–117 (1993).
Jones, T. A., Zou, J. Y., Cowan, S. W. & Kjeldgaard, M. Acta crystallogr. A47, 110–119 (1991).
Brünger, A. T. XPLOR version 3.1: a system for X-ray crystallography and NMR (Yale University Press, New Haven, 1993).
Read, R. J. Acta crystallogr. A42, 140–149 (1986).
Müller, C. W., Rey, F. A., Sodeoka, M., Verdine, G. L. & Harrison, S. C. Nature 373, 311–317 (1995).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ghosh, G., Duyne, G., Ghosh, S. et al. Structure of NF-κB p50 homodimer bound to a κB site. Nature 373, 303–310 (1995). https://doi.org/10.1038/373303a0
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1038/373303a0
This article is cited by
-
Exploration of the mechanism by which Huangqi Guizhi Wuwu decoction inhibits Lps-induced inflammation by regulating macrophage polarization based on network pharmacology
BMC Complementary Medicine and Therapies (2023)
-
Backbone and side-chain chemical shift assignments of p50 subunit of NF-κB transcription factor
Biomolecular NMR Assignments (2021)
-
Backbone resonance assignments of the dimeric domain of the p50 NF-kappaB subunit
Biomolecular NMR Assignments (2020)
-
Insights into the assembly and architecture of a Staufen-mediated mRNA decay (SMD)-competent mRNP
Nature Communications (2019)
-
DNA Binding and Phosphorylation Regulate the Core Structure of the NF-κB p50 Transcription Factor
Journal of the American Society for Mass Spectrometry (2019)