Abstract
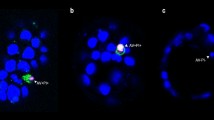
Phagocytosis of apoptotic cells is required to prevent tissue injury. Professional phagocytes, such as monocyte-derived macrophages, are highly efficient scavengers of apoptotic cells but their presence cannot always be relied on; in that case, removal of effete cells is accomplished by helpful neighbours. This study describes differences in the efficiency with which apoptotic cells of the same type, but dying in response to different triggers, are engulfed; this varies from engulfment that is so proficient few or no unengulfed apoptotic cells are found, to engulfment that is so delayed apoptotic cells have become secondarily necrotic at the point of engulfment. In all cases the efficiency of engulfment is determined at least in part by the dying cells themselves. p53- and Bax-transfected kidney epithelial (293) cells (transiently transfected using a non-toxic method) were engulfed so proficiently by homotypic neighbours that cells did not show evidence of engagement of the apoptotic programme (chromatin condensation and TUNEL positivity) until engulfment had taken place. Engulfment nonetheless required activation of at least initiator caspases. 293 cells induced to apoptose by other means (etoposide and staurosporine treatment) were not so efficiently ingested: unengulfed apoptotic cells were consistently revealed at all doses and time points, even when treated cells were mixed with healthy, non-treated 293 cells. These data make it extremely unlikely that the fraction of viable, unaffected neighbours determines the efficiency with which engulfment proceeds. Furthermore, 293 cells treated with etoposide or staurosporine were differentially appealing both to homotypic neighbours and to cells in the professional phagocyte lineage (THP-1 cells). If different apoptotic stimuli programme cells to be recognised with different efficiencies, pathways to apoptosis may be injury limiting to greater or lesser degrees. Cell Death and Differentiation (2001) 8, 734–746
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- CMTMR:
-
5-(and 6)-(((4-chloromethyl)benzoyl)amino)tetramethylrhodamine
- CMFDA:
-
5-chloromethylfluorescein diacetate
- DIC:
-
differential interference contrast
- GFP:
-
green fluorescent protein
- PI:
-
propidium iodide
- PS:
-
phosphatidylserine
- TUNEL:
-
terminal deoxynucleotide transferase-mediated dUTP-fluorescein nick-end labelling
- ZVAD-fmk:
-
benzyloxycarbonyl-valinyl-alanyl-aspartyl-(OMe)-fluoromethyl ketone
References
Raff MC . 1992 Social controls on cell survival and cell death Nature 356: 397–400
Ishizaki Y, Cheng L, Mudge AW, Raff MC . 1995 Programmed cell death by default in embryonic cells, fibroblasts, and cancer cells Mol. Biol. Cell 6: 1443–1458
Weil M, Jacobson MD, Coles HS, Davies TJ, Gardner RL, Raff KD, Raff MC . 1996 Constitutive expression of the machinery for programmed cell death J. Cell Biol. 133: 1053–1059
Savill J . 1997 Recognition and phagocytosis of cells undergoing apoptosis Br. Med. Bull. 53: 491–508
Platt N, da Silva R, Gordon S . 1998 Recognizing death: the phagocytosis of apoptotic cells Trends Cell Biol. 8: 365–372
Wyllie AH, Kerr JFR, Currie AR . 1980 Cell death: the significance of apoptosis Int. Rev. Cytol. 68: 251–306
Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y, Itoh N, Suda T, Nagata S . 1993 Lethal effect of the anti-Fas antibody in mice Nature 364: 806–809
Ellis RE, Jacobson DM, Horvitz HR . 1991 Genes required for the engulfment of cell corpses during programmed cell death in Caenorhabditis elegans Genetics 129: 70–94
Wu Y-C, Horvitz R . 1998 C. elegans phagocytosis and cell-migration protein CED-5 is similar to human DOCK180 Nature 392: 501–504
Liu QA, Hengartner MO . 1998 Candidate adaptor protein CED-6 promotes the engulfment of apoptotic cells in C. elegans Cell 93: 961–972
Wu Y-C, Horvitz R . 1998 The C. elegans cell corpse engulfment gene ced-7 encodes a protein similar to ABC transporters Cell 93: 951–960
Gobé GC, Axelson RA . 1987 Genesis of renal tubular atrophy in experimental hydronephrosis in the rat Role of apoptosis. Lab. Invest. 56: 273–282
Dini L, Lentini A, Diez GD, Rocha M, Falasca L, Serafino L, Vidal-Vanaclocha F . 1995 Phagocytosis of apoptotic bodies by liver endothelial cells J. Cell Sci. 108: 967–973
Hess KL, Tudor K-SRS, Johnson JD, Osati-Ashtiani F, Askew DS, Cook-Mills JM . 1997 Human and murine high endothelial venule cells phagocytose apoptotic leukocytes Exp. Cell Res. 236: 404–411
Savill J, Smith J, Ren Y, Sarraf C, Abbott F, Rees AJ . 1992 Glomerular mesangial cells and inflammatory macrophages ingest neutrophils undergoing apoptosis Kidney Int. 42: 924–936
Baker AJ, Mooney A, Hughes J, Lombardi D, Johnson RJ, Savill J . 1994 Mesangial cell apoptosis: the major mechanism for resolution of glomerular hypercellularity in experimental mesangial proliferative nephritis J. Clin. Invest. 94: 2105–2116
Hall SE, Savill J, Henson PM, Haslett C . 1994 Apoptotic neutrophils are phagocytosed by fibroblasts with participation of the fibroblast vitronectin receptor and involvement of a mannose/fucose-specific lectin J. Immunol. 153: 3218–3227
Duvall E, Wyllie AH, Morris RG . 1985 Macrophage recognition of cells undergoing programmed cell death Immunology 56: 351–358
Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM . 1992 Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages J. Immunol. 148: 2207–2216
Verhoeven B, Schlegel RA, Williamson P . 1995 Mechanisms of phosphatidylserine exposure, a phagocyte recognition signal, on apoptotic T lymphocytes J. Exp. Med. 182: 1597–1601
Marguet D, Luciani M-F, Moynault A, Williamson P, Chimini G . 1999 Engulfment of apoptotic cells involves the redistribution of membrane phosphatidylserine on phagocyte and prey Nature Cell Biol. 1: 454–456
Flora PK, Gregory CD . 1995 Recognition pathways in the interaction of macrophages with apoptotic B cells In Leukocyte typing: white cell differentiation antigens, Schlossman SF, Boumsell L and Gilks W, eds Oxford: Oxford University Press p 1675
Moffat OD, Devitt A, Bell ED, Simmons DL, Gregory CD . 1999 Macrophage recognition of ICAM-3 on apoptotic leukocytes J. Immunol. 162: 6800–6810
Savill J, Dransfield I, Hogg N, Haslett C . 1990 Vitronectin receptor-mediated phagocytosis of cells undergoing apoptosis Nature 343: 170–173
Rubartelli A, Poggi A, Zocchi MR . 1997 The selective engulfment of apoptotic bodies by dendritic cells is mediated by the αvβ3 integrin and requires intracellular and extracellular calcium Eur. J. Immunol. 27: 1893–1900
Savill J, Hogg N, Ren Y, Haslett C . 1992 Thrombospondin cooperates with CD36 and the vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis J. Clin. Invest. 90: 1513–1522
Ren Y, Silverstein RL, Allen J, Savill J . 1995 CD36 gene transfer confers capacity for phagocytosis of cells undergoing apoptosis J. Exp. Med. 181: 1857–1862
Platt N, Suzuki H, Kurihara Y, Kodama T, Gordon S . 1996 Role for the class A macrophage scavenger receptor in the phagocytosis of apoptotic thymocytes in vitro Proc. Natl. Acad. Sci. USA 93: 12456–12460
Luciani M-F, Chimini G . 1996 The ATP binding cassette transporter ABC1 is required for the engulfment of corpses generated by apoptotic cell death EMBO J. 15: 226–236
Moynault A, Luciani M-F, Chimini G . 1998 ABC1, the mammalian homologue of the engulfment gene ced-7, is required during phagocytosis of both necrotic and apoptotic cells Biochem. Soc. Transactions 26: 629–635
Hamon Y, Broccardo C, Chambenoit O, Luciani M-F, Toti F, Chaslin S, Freyssinet J-M, Devaux PF, McNeish J, Marguet D, Chimini G . 2000 ABC1 promotes engulfment of apoptotic cells and transbilayer redistribution of phosphatidylserine Nature Cell Biol. 2: 399–406
Devitt A, Moffat OD, Raykundalia C, Capra JD, Simmons DL, Gregory CD . 1998 Human CD14 mediates recognition and phagocytosis of apoptotic cells Nature 392: 505–509
Korb LC, Ahearn JM . 1997 C1q binds directly and specifically to surface blebs of apoptotic human keratinocytes J. Immunol. 158: 4525–4528
Botto M, Dell'Agnola C, Bygrave AE, Thompson EM, Cook HT, Petry F, Loos M, Pandolfi PP, Walport MJ . 1998 Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies Nature Genet. 19: 56–59
Fadok VA, Bratton DL, Rose DM, Pearson A, Ezekewitz RAB, Henderson PM . 2000 A receptor for phosphatidylserine-specific clearance of apoptotic cells Nature 405: 85–90
Debbas, White E . 1993 Wild-type p53 mediates apoptosis by E1A, which is inhibited by E1B Genes Dev. 7: 546–554
Pan G, O'Rourke K, Dixit VM . 1998 Caspase-9, Bcl-XL and Apaf-1 form a ternary complex J. Biol. Chem. 273: 5841–5845
Han J, Wallen HD, Nunez G, White E . 1998 E1B 19,000-molecular-weight protein interacts with and inhibits CED-4-dependent, FLICE-mediated apoptosis Mol. Cell. Biol. 18: 6052–6062
Jürgensmeier JM, Zhihua X, Deveraux Q, Ellerby L, Bredesen D, Reed JC . 1998 Bax directly induces release of cytochrome c from isolated mitochondria Proc. Natl. Acad. Sci. USA 95: 4997–5002
Desagher S, Osen Sand A, Nichols A, Eskes R, Montessuit S, Lauper S, Maundrell K, Antonsson B, Martinou JC . 1999 J. Cell Biol. 144: 891–901
Zhuang J, Ren Y, Snowden RT, Zhu H, Gogvadze V, Savill J, Cohen GM . 1998 Dissociation of phagocyte recognition of cells undergoing apoptosis from other features of the apoptotic program J. Biol. Chem. 273: 15628–15632
Vogelstein B, Lane D, Levine A . 2000 Surfing the p53 network Nature 408: 307–310
van Maanen JMS, Retèl J, de Vries J, Pinedo HM . 1988 Mechanisms of action of antitumour drug etoposide: a review J. Natl. Cancer Inst. 80: 1526–1533
Jacobson MD, Burne JF, Raff MC . 1994 Programmed cell death and Bcl-2 protection in the absence of a nucleus EMBO J. 13: 1899–1910
Fadok VA, Savill J, Haslett C, Doherty DE, Bratton DL, Campbell PA, Henson PM . 1992 Different populations of macrophages use either the vitronectin receptor or the phosphatidylserine receptor to recognise and remove apoptotic cells J. Immunol. 149: 4029–4035
Tsuchiya S, Kobayashi Y, Goto Y, Okumura H, Nakae S, Konno T, Keiya T . 1982 Induction of maturation in cultured human monocytic leukemia cells by a phorbol diester Cancer Res. 42: 1530–1536
Raff MC, Barres BA, Burne JF, Coles HS, Ishizaki Y, Jacobson MD . 1993 Programmed cell death and the control of cell survival: lessons from the nervous system Science 262: 695–700
McCarthy NJ, Whyte MKB, Gilbert CS, Evans GI . 1997 Inhibition of Ced-3/ICE-related proteases does not prevent cell death induced by oncogenes, DNA damage, or the Bcl-2 homologue Bak J. Cell Biol. 136: 215–227
Jacobson MD, Weil M, Raff MC . 1996 Role of Ced-3/ICE-family proteases in staurosporine-induced programmed cell death J. Cell Biol. 133: 1041–1051
Thornberry NA, Lazebnik Y . 1998 Caspases: enemies within Science 281: 1312–1316
Knepper-Nicolai B, Savill J, Brown S . 1998 Constitutive apoptosis in human neutrophils requires synergy between calpains and the proteasome downstream of caspases J. Biol. Chem. 273: 30530–30536
Gross A, Jockel J, Wei MC, Korsmeyer SJ . 1998 Enforced dimerization of Bax results in its translocation, mitochondrial dysfunction and apoptosis EMBO J. 17: 3878–3885
Nechushtan A, Smith CL, Hsu Y-T, Youle RJ . 1999 Conformation of the Bax C-terminus regulates subcellular location and cell death EMBO J. 18: 2330–2341
Porter AG . 1999 Protein translocation in apoptosis Trends Cell Biol. 9: 394–401
Ausubel FA, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, eds. 1998 Current protocols in molecular biology New York: John Wiley & Sons
Ryan KM, Vousden KH . 1998 Characterization of structural p53 mutants, which show selective defects in apoptosis but not cell cycle, arrest Mol. Cell. Biol. 18: 3692–3698
Oltvai ZN, Milliman CL, Korsmeyer J . 1993 Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death Cell 74: 609–619
Acknowledgements
We would like to thank Carol Midgeley, David Lane and other members of the laboratory for generous provision of the cell line Saos2::p53teti, p53 expression vectors, and p53 antibodies. Furthermore we thank K Ryan and K Vousden for kindly providing us with pcDNA3/p53, and A Chen and CB Thompson for the generous gift of psFFVneo-Bax. We are grateful to Simon Brown and Niall McTavish for helpful discussion. This work was funded by the Wellcome Trust grant no. 033790. B Spruce is a Wellcome Senior Research Fellow in Clinical Science.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by K Vousden
Rights and permissions
About this article
Cite this article
Wiegand, U., Corbach, S., Prescott, A. et al. The trigger to cell death determines the efficiency with which dying cells are cleared by neighbours. Cell Death Differ 8, 734–746 (2001). https://doi.org/10.1038/sj.cdd.4400867
Received:
Revised:
Accepted:
Issue date:
DOI: https://doi.org/10.1038/sj.cdd.4400867
Keywords
This article is cited by
-
Secondary necrosis in multicellular animals: an outcome of apoptosis with pathogenic implications
Apoptosis (2008)
-
Rapid Hair Cell Loss: A Mouse Model for Cochlear Lesions
Journal of the Association for Research in Otolaryngology (2008)
-
Proenkephalin assists stress-activated apoptosis through transcriptional repression of NF-κB- and p53-regulated gene targets
Cell Death & Differentiation (2007)
-
Macrophages use different internalization mechanisms to clear apoptotic and necrotic cells
Cell Death & Differentiation (2006)
-
Immunology of Apoptosis and Necrosis
Biochemistry (Moscow) (2005)