Abstract
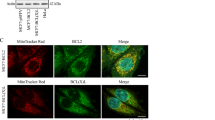
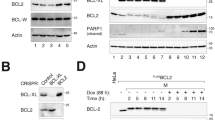
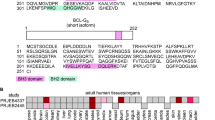
Proteins of the Bcl-2 family regulate apoptosis, some antagonizing cell death and others, such as Bcl-xS, promoting it. We previously showed that expression of Bcl-xS in PC12 cells is a useful system for studying the mechanism of Bcl-xS-induced apoptosis. To further investigate this apoptotic effect and its prevention by anti-apoptotic agents, we assessed the role of distinct Bcl-xS domains, via the study of their mutations, on the ability of Bcl-xS to induce apoptosis and to localize to the mitochondria, as well as the ability of these domains to counteract the effects of anti-apoptotic agents on Bcl-xS. Deletion of the transmembrane domain (ΔTM) prevented the localization of Bcl-xS ΔTM to the mitochondria and the ability of this mutant to induce apoptosis. Deletion of the amino acids GD 94–95 from the BH3 domain, or deletion of the loop region, impaired the ability of these mutants to induce apoptosis but not their localization to the mitochondria. Deletion of the BH4 domain or destruction of the caspase cleavage site in the loop region (by replacing amino acid D61 with A61) did not affect either the localization of these mutants to the mitochondria or their ability to induce cell death. It thus appears that Bcl-xS-induced apoptosis in PC12 cells is mediated by localization of Bcl-xS to the mitochondria by a process that requires the transmembrane domain. Furthermore, once localized to the mitochondria Bcl-xS requires the BH3 domain, and to a lesser extent the loop domain, for its subsequent activity. The anti-apoptotic agents Bcl-2 and Bcl-xL, the caspase inhibitor Z-VAD-FMK, and nerve growth factor (NGF) did not prevent Bcl-xS localization to the mitochondria, and did not require the BH4 or the loop domains of Bcl-xS for their survival effect. Bcl-xS is capable of forming homodimers with itself and heterodimers with Bcl-xL or Bcl-2. Accordingly co-expression of Bcl-xS ΔTM with Bcl-xS, Bcl-2, or Bcl-xL leads to a change in the subcellular distribution of Bcl-xS ΔTM, from a diffuse distribution throughout the cell to a more defined distribution. Moreover co-immunoprecipitation experiments directly demonstrated that Bcl-xS can associate with GFP-Bcl-xS, Bcl-xL, or Bcl-2. These results suggest that such Bcl-xS interactions may be important for the mechanism of action of this protein. Cell Death and Differentiation (2001) 8, 933–942
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- NGF:
-
nerve growth factor
- PBS:
-
phosphate-buffered saline
- Z-VAD-FMK:
-
benzyloxycarbonyl-Val-Ala-Asp-fluoro-methylketone
- TM:
-
transmembrane
- SEAP:
-
secreted alkaline phosphatase
- WT:
-
wild-type
- PCR:
-
polymerase chain reaction
- TBS:
-
Tris-buffered saline
References
Vaux DL, Korsmeyer SJ . 1999 Cell death in development Cell 96: 245–254
Evan G, Littlewood T . 1998 A matter of life and cell death Science 281: 1317–1322
Salvesen GS, Dixit VM . 1997 Caspases: intracellular signaling by proteolysis Cell 91: 443–446
Thornberry NA, Lazebnik Y . 1998 Caspases: enemies within Science 281: 1312–1316
Gross A, McDonnell JM, Korsmeyer SJ . 1999 BCL-2 family members and the mitochondria in apoptosis Genes Dev. 13: 1899–1911
Kelekar A, Thompson CB . 1998 Bcl-2-family proteins: the role of the BH3 domain in apoptosis Trends Cell Biol. 8: 324–330
Reed JC . 1998 Bcl-2 family proteins Oncogene 17: 3225–3236
Sedlak TW, Oltvai ZN, Yang E, Wang K, Boise LH, Thompson CB, Korsmeyer SJ . 1995 Multiple Bcl-2 family members demonstrate selective dimerizations with Bax Proc. Natl. Acad. Sci. USA 92: 7834–7838
Yin XM, Oltvai ZN, Korsmeyer SJ . 1994 BH1 and BH2 domains of Bcl-2 are required for inhibition of apoptosis and heterodimerization with Bax [see comments] Nature 369: 321–323
Oltvai ZN, Korsmeyer SJ . 1994 Checkpoints of dueling dimers foil death wishes [comment] Cell 79: 189–192
Oltvai ZN, Milliman CL, Korsmeyer SJ . 1993 Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death Cell 74: 609–619
Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Eberstadt M, Yoon HS, Shuker SB, Chang BS, Minn AJ, Thompson CB, Fesik SW . 1997 Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis Science 275: 983–986
Boise LH, Gonzalez-Garcia M, Postema CE, Ding L, Lindsten T, Turka LA, Mao X, Nunez G, Thompson CB . 1993 bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death Cell 74: 597–608
Wang HG, Miyashita T, Takayama S, Sato T, Torigoe T, Krajewski S, Tanaka S, Hovey 3rd L, Troppmair J, Rapp UR, Reed JC . 1994 Apoptosis regulation by interaction of Bcl-2 protein and Raf-1 kinase Oncogene 9: 2751–2756
Shibasaki F, Kondo E, Akagi T, McKeon F . 1997 Suppression of signalling through transcription factor NF-AT by interactions between calcineurin and Bcl-2 Nature 386: 728–731
Huang DC, Adams JM, Cory S . 1998 The conserved N-terminal BH4 domain of Bcl-2 homologues is essential for inhibition of apoptosis and interaction with CED-4 EMBO J. 17: 1029–1039
Muchmore SW, Sattler M, Liang H, Meadows RP, Harlan JE, Yoon HS, Nettesheim D, Chang BS, Thompson CB, Wong SL, Ng SL, Fesik SW . 1996 X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death Nature 381: 335–341
Chang BS, Minn AJ, Muchmore SW, Fesik SW, Thompson CB . 1997 Identification of a novel regulatory domain in Bcl-X(L) and Bcl-2 EMBO J. 16: 968–977
Haldar S, Chintapalli J, Croce CM . 1996 Taxol induces bcl-2 phosphorylation and death of prostate cancer cells Cancer Res. 56: 1253–1255
Clem RJ, Cheng EH, Karp CL, Kirsch DG, Ueno K, Takahashi A, Kastan MB, Griffin DE, Earnshaw WC, Veliuona MA, Hardwick JM . 1998 Modulation of cell death by Bcl-XL through caspase interaction Proc. Natl. Acad. Sci. USA 95: 554–559
Nguyen M, Millar DG, Yong VW, Korsmeyer SJ, Shore GC . 1993 Targeting of Bcl-2 to the mitochondrial outer membrane by a COOH-terminal signal anchor sequence J. Biol. Chem. 268: 25265–25268
Nguyen M, Branton PE, Walton PA, Oltvai ZN, Korsmeyer SJ, Shore GC . 1994 Role of membrane anchor domain of Bcl-2 in suppression of apoptosis caused by E1B-defective adenovirus J. Biol. Chem. 269: 16521–16524
Zha H, Fisk HA, Yaffe MP, Mahajan N, Herman B, Reed JC . 1996 Structure-function comparisons of the proapoptotic protein Bax in yeast and mammalian cells Mol. Cell. Biol. 16: 6494–6508
Li H, Yuan J . 1999 Deciphering the pathways of life and death Curr. Opin. Cell Biol. 11: 261–266
Gonzalez-Garcia M, Perez-Ballestero R, Ding L, Duan L, Boise LH, Thompson CB, Nunez G . 1994 bcl-XL is the major bcl-x mRNA form expressed during murine development and its product localizes to mitochondria Development 120: 3033–3042
Dixon EP, Stephenson DT, Clemens JA, Little SP . 1997 Bcl-Xshort is elevated following severe global ischemia in rat brains Brain Res. 776: 222–229
Heermeier K, Benedict M, Li M, Furth P, Nunez G, Hennighausen L . 1996 Bax and Bcl-xs are induced at the onset of apoptosis in involuting mammary epithelial cells Mech. Dev. 56: 197–207
Clarke MF, Apel IJ, Benedict MA, Eipers PG, Sumantran V, Gonzalez-Garcia M, Doedens M, Fukunaga N, Davidson B, Dick JE, Minn AJ, Boise LH, Thompson CB, Wicha M, Nunez G . 1995 A recombinant bcl-x s adenovirus selectively induces apoptosis in cancer cells but not in normal bone marrow cells Proc. Natl. Acad. Sci. USA 92: 11024–11028
Ealovega MW, McGinnis PK, Sumantran VN, Clarke MF, Wicha MS . 1996 bcl-xs gene therapy induces apoptosis of human mammary tumors in nude mice Cancer Res. 56: 1965–1969
Dole MG, Clarke MF, Holman P, Benedict M, Lu J, Jasty R, Eipers P, Thompson CB, Rode C, Bloch C, Nunez, Castle VP . 1996 Bcl-xS enhances adenoviral vector-induced apoptosis in neuroblastoma cells Cancer Res. 56: 5734–5740
Lindenboim L, Yuan J, Stein R . 2000 Bcl-xS and Bax induce different apoptotic pathways in PC12 cells [In Process Citation] Oncogene 19: 1783–1793
Haviv R, Lindenboim L, Yuan J, Stein R . 1998 Need for caspase-2 in apoptosis of growth-factor-deprived PC12 cells J. Neurosci. Res. 52: 491–497
Stefanis L, Troy CM, Qi H, Shelanski ML, Greene LA . 1998 Caspase-2 (Nedd-2) processing and death of trophic factor-deprived PC12 cells and sympathetic neurons occur independently of caspase-3 (CPP32)-like activity J. Neurosci. 18: 9204–9215
Chang BS, Kelekar A, Harris MH, Harlan JE, Fesik SW, Thompson CB . 1999 The BH3 domain of Bcl-x(S) is required for inhibition of the antiapoptotic function of Bcl-x(L) Mol. Cell. Biol. 19: 6673–6681
Chen G, Cizeau J, Vande Velde C, Park JH, Bozek G, Bolton J, Shi L, Dubik D, Greenberg A . 1999 Nix and nip3 form a subfamily of pro-apoptotic mitochondrial proteins [In Process Citation] J. Biol. Chem. 274: 7–10
Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ . 1997 Movement of Bax from the cytosol to mitochondria during apoptosis J. Cell Biol. 139: 1281–1292
Hegde R, Srinivasula SM, Ahmad M, Fernandes-Alnemri T, Alnemri ES . 1998 Blk, a BH3-containing mouse protein that interacts with Bcl-2 and Bcl-xL, is a potent death agonist J. Biol. Chem. 273: 7783–7786
Kelekar A, Chang BS, Harlan JE, Fesik JW, Thompson CB . 1997 Bad is a BH3 domain-containing protein that forms an inactivating dimer with Bcl-XL Mol. Cell. Biol. 17: 7040–7046
Wang K, Yin XM, Chao DT, Milliman CL, Korsmeyer SJ . 1996 BID: a novel BH3 domain-only death agonist Genes Dev. 10: 2859–2869
Boyd JM, Gallo GJ, Elangovan B, Houghton AB, Malstrom S, Avery BJ, Ebb RG, Subramanian T, Chittenden T, Lutz RJ, Chinnadurai G . 1995 Bik, a novel death-inducing protein shares a distinct sequence motif with Bcl-2 family proteins and interacts with viral and cellular survival-promoting proteins Oncogene 11: 1921–1928
Li H, Zhu H, Xu CJ, Yuan J . 1998 Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis Cell 94: 491–501
de Moissac D, Zheng H, Kirshenbaum LA . 1999 Linkage of the BH4 domain of Bcl-2 and the nuclear factor kappaB signaling pathway for suppression of apoptosis J. Biol. Chem. 274: 29505–29509
Fang G, Chang BS, Kim CN, Perkins C, Thompson CB, Bhalla KN . 1998 “Loop” domain is necessary for taxol-induced mobility shift and phosphorylation of Bcl-2 as well as for inhibiting taxol-induced cytosolic accumulation of cytochrome c and apoptosis Cancer Res. 58: 3202–3208
Eskes R, Desagher S, Antonsson B, Martinou JC . 2000 Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane Mol. Cell. Biol. 20: 929–935
Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR . 1989 Site-directed mutagenesis by overlap extension using the polymerase chain reaction [see comments] Gene 77: 51–59
Berger J, Hauber J, Hauber R, Geiger R, Cullen BR . 1998 Secreted placental alkaline phosphatase: a powerful new quantitative indicator of gene expression in eukaryotic cells Gene 66: 1–10
Acknowledgements
We thank Dr Craig Thompson for providing the Bcl-xL and Bcl-xS vectors, and Dr Stanley Korsmeyer for providing Bcl-2 vector. We are grateful to Ms Shirley Smith for excellent editorial assistance. This work was supported by the United States–Israel Binational Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by S Korsmeyer
Rights and permissions
About this article
Cite this article
Lindenboim, L., Borner, C. & Stein, R. Bcl-xS can form homodimers and heterodimers and its apoptotic activity requires localization of Bcl-xS to the mitochondria and its BH3 and loop domains. Cell Death Differ 8, 933–942 (2001). https://doi.org/10.1038/sj.cdd.4400888
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.cdd.4400888
Keywords
This article is cited by
-
Non-canonical function of Bax in stress-induced nuclear protein redistribution
Cellular and Molecular Life Sciences (2013)
-
Disruption of the VDAC2–Bak interaction by Bcl-xS mediates efficient induction of apoptosis in melanoma cells
Cell Death & Differentiation (2012)
-
Determining the impact of alternative splicing events on transcriptome dynamics
BMC Research Notes (2008)
-
Bak but not Bax is essential for Bcl-xS-induced apoptosis
Cell Death & Differentiation (2005)
-
Mechanism of mitochondrial stress-induced resistance to apoptosis in mitochondrial DNA-depleted C2C12 myocytes
Cell Death & Differentiation (2005)