Abstract
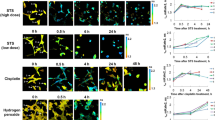
Mitochondria play central roles in cellular metabolism and apoptosis and are a major source of reactive oxygen species (ROS). We investigated the role of ROS and mitochondria in radiation-induced apoptosis in multiple myeloma cells. Two distinct levels of ROS were generated following irradiation: a small increase observed early, and a pronounced late increase, associated with depletion of reduced glutathione (GSH) and collapse of mitochondrial membrane potential (Δψm). Exogenous ROS and caspase-3 induced Δψm drop and cytochrome c release from mitochondria, which could be prevented by molecular (dominant-negative caspase-9) and pharmacologic (zVAD-fmk) caspase inhibitors and overexpression of Bcl-2. Exogenous ROS also induced mitochondrial permeability transition (PT) pore opening and cytochrome c release in isolated mitochondria, which could be blocked by inhibition of PT with cyclosporin A. These results indicate that the late ROS production is associated with increased PT pore opening and decreased Δψm, and GSH, events associated with caspase activation and cytochrome c release.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- cyt c:
-
cytochrome c
- CM-H2:
-
DCFDA
- 2′:
-
7′-dichlorodihydrofluorescein diacetate
- DEVD:
-
acetyl-asp-glu-val-asp
- DHE:
-
dihydroethidium
- fmk:
-
fluoromethyl ketone
- GSH:
-
reduced glutathione
- GSSG:
-
oxidized glutathione
- H2O2:
-
hydrogen peroxide
- IR:
-
ionizing radiation
- mAb:
-
monoclonal antibody
- PI:
-
propidium iodide
- PBS:
-
phosphate-buffered saline
- PT:
-
mitochondrial permeability transition
- ROS:
-
reactive oxygen species
- YVAD:
-
acetyl-Tyr-Val-Ala-Asp
- zVAD:
-
Benzyloxycarbonyl-Val-Ala-Asp
- Δψ m :
-
mitochondrial membrane potential
References
Wang X (2001) The expanding role of mitochondria in apoptosis. Genes Dev. 15: 2922–2933
Du C, Fang M, Li Y, Li L and Wang X (2000) Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 102: 33–42
Joza N, Susin SA, Daugas E, Stanford WL, Cho SK, Li CY, Sasaki T, Elia AJ, Cheng HY, Ravagnan L, Ferri KF, Zamzami N, Wakeham A, Hakem R, Yoshida H, Kong YY, Mak TW, Zuniga-Pflucker JC, Kroemer G and Penninger JM (2001) Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature 410: 549–554
Li LY, Luo X and Wang X (2001) Endonuclease G is an apoptotic DNase when released from mitochondria. Nature 412: 95–99
Li W, Srinivasula SM, Chai J, Li P, Wu JW, Zhang Z, Alnemri ES and Shi Y (2002) Structural insights into the pro-apoptotic function of mitochondrial serine protease HtrA2/Omi. Nat. Struct. Biol. 22: 22
Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES and Wang X (1997) Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91: 479–489
Zou H, Henzel WJ, Liu X, Lutschg A and Wang X (1997) Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell 90: 405–413
Thornberry NA and Lazebnik Y (1998) Caspases: enemies within. Science 281: 1312–1316
Voehringer DW, McConkey DJ, McDonnell TJ, Brisbay S and Meyn RE (1998) Bcl-2 expression causes redistribution of glutathione to the nucleus. Proc. Natl. Acad. Sci. USA 95: 2956–2960
Hockenbery DM, Oltvai ZN, Yin XM, Milliman CL and Korsmeyer SJ (1993) Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell 75: 241–251
Kane DJ, Sarafian TA, Anton R, Hahn H, Gralla EB, Valentine JS, Ord T and Bredesen DE (1993) Bcl-2 inhibition of neural death: decreased generation of reactive oxygen species. Science 262: 1274–1277
Voehringer DW (1999) BCL-2 and glutathione: alterations in cellular redox state that regulate apoptosis sensitivity. Free Radic. Biol. Med. 27: 945–950
Steinman HM (1995) The Bcl-2 oncoprotein functions as a pro-oxidant. J. Biol. Chem. 270: 3487–3490
Kluck R, Bossy-Wetzel E, Green D and Newmeyer D (1997) The release of cytochrome c from mitochondria: A primary site for Bcl-2 regulation of apoptosis. Science 275: 1132–1136
Yang J, Liu X, Bhalla K, Kim C, Ibrado A-M, Cai J, Peng T-I, Jones DP and Wang X (1997) Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science 275: 1129–1132
Vander Heiden MG, Chandel NS, Williamson EK, Schumacker PT and Thompson CB (1997) Bcl-xL regulates the membrane potential and volume homeostasis of mitochondria. Cell 91: 627–637
Vander Heiden MG and Thompson CB (1999) Bcl-2 proteins: regulators of apoptosis or of mitochondrial homeostasis? Nat. Cell Biol. 1: E209–216
Marzo I, Brenner C, Zamzami N, Jurgensmeier JM, Susin SA, Vieira HL, Prevost MC, Xie Z, Matsuyama S, Reed JC and Kroemer G (1998) Bax and adenine nucleotide translocator cooperate in the mitochondrial control of apoptosis. Science 281: 2027–2031
Petit PX, Lecoeur H, Zorn E, Dauguet C, Mignotte B and Gougeon ML (1995) Alterations in mitochondrial structure and function are early events of dexamethasone-induced thymocyte apoptosis. J. Cell Biol. 130: 157–167
Slater AF, Nobel CS and Orrenius S (1995) The role of intracellular oxidants in apoptosis. Biochim. Biophys. Acta. 1271: 59–62
Jacobson MD and Raff MC (1995) Programmed cell death and Bcl-2 protection in very low oxygen. Nature 374: 814–816
Goossens V, Grooten J, De Vos K and Fiers W (1995) Direct evidence for tumor necrosis factor-induced mitochondrial reactive oxygen intermediates and their involvement in cytotoxicity. Proc. Natl. Acad. Sci. USA 92: 8115–8119
Li PF, Dietz R and von Harsdorf R (1999) p53 regulates mitochondrial membrane potential through reactive oxygen species and induces cytochrome c-independent apoptosis blocked by Bcl-2. EMBO. J. 18: 6027–6036
Polyak K, Xia Y, Zweier JL, Kinzler KW and Vogelstein B (1997) A model for p53-induced apoptosis. Nature 389: 300–305
Wong GH and Goeddel DV (1988) Induction of manganous superoxide dismutase by tumor necrosis factor: possible protective mechanism. Science 242: 941–944
Melov S, Coskun P, Patel M, Tuinstra R, Cottrell B, Jun AS, Zastawny TH, Dizdaroglu M, Goodman SI, Huang TT, Miziorko H, Epstein CJ and Wallace DC (1999) Mitochondrial disease in superoxide dismutase 2 mutant mice. Proc. Natl. Acad. Sci. USA 96: 846–851
Chen Q, Gong B and Almasan A (2000) Distinct stages of cytochrome c release from mitochondria: evidence for a feedback amplification loop linking caspase activation to mitochondrial dysfunction in genotoxic stress induced apoptosis. Cell Death Differ. 7: 227–233
Chen Q, Gong B, Mahmoud-Ahmed A, Zhou A, Hsi ED, Hussein M and Almasan A (2001) Apo2L/TRAIL and Bcl-2-related proteins regulate type I interferon- induced apoptosis in multiple myeloma. Blood 98: 2183–2192
Almasan A, Yin Y, Kelly RE, Lee EY, Bradley A, Li W-W, Bertino JR and Wahl GM (1995) Deficiency of retinoblastoma protein leads to inappropriate S-phase entry, activation of E2F-responsive genes, and apoptosis. Proc. Natl. Acad. Sci. USA 92: 5436–5440
Haliwell B and Gutteridge JMC (1989) In Free Radicals in Biology and Medicine, 2 ed., Oxford: Clarendon Press, pp 1–81
Gong B, Chen Q, Endlich B, Mazumder S and Almasan A (1999) Ionizing radiation-induced, Bax-mediated cell death is dependent on activation of serine and cysteine proteases. Cell Growth Differ. 10: 491–502
Armstrong JS, Steinauer KK, Hornung B, Irish JM, Lecane P, Birrell GW, Peehl DM and Knox SJ (2002) Role of glutathione depletion and reactive oxygen species generation in apoptotic signaling in a human B lymphoma cell line. Cell Death Differ. 9: 252–263
Higuchi M, Honda T, Proske RJ and Yeh ET (1998) Regulation of reactive oxygen species-induced apoptosis and necrosis by caspase 3-like proteases. Oncogene 17: 2753–2760
Ott M, Robertson JD, Gogvadze V, Zhivotovsky B and Orrenius S (2002) Cytochrome c release from mitochondria proceeds by a two-step process. Proc. Natl. Acad. Sci. USA 99: 1259–1263
Xia T, Jiang C, Li L, Wu C, Chen Q and Liu SS (2002) A study on permeability transition pore opening and cytochrome c release from mitochondria, induced by caspase-3 in vitro. FEBS Lett. 510: 62–66
Boveris A and Chance B . (1973) The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 134: 707–716
Cai J and Jones DP . (1998) Superoxide in apoptosis. Mitochondrial generation triggered by cytochrome c loss. J. Biol. Chem. 273: 11401–11404
Tan S, Sagara Y, Liu Y, Maher P and Schubert D (1998) The regulation of reactive oxygen species production during programmed cell death. J. Cell. Biol. 141: 1423–1432
Green D and Kroemer G (1998) The central executioners of apoptosis: caspases or mitochondria? Trends Cell Biol. 8: 267–271
Susin SA, Zamzami N, Castedo M, Daugas E, Wang HG, Geley S, Fassy F and Reed JC, Kroemer G (1997) The central executioner of apoptosis: multiple connections between protease activation and mitochondria in Fas/APO-1/CD95- and ceramide-induced apoptosis. J. Exp. Med. 186: 25–37
Goldstein JC, Waterhouse NJ, Juin P, Evan GI and Green DR (2000) The coordinate release of cytochrome c during apoptosis is rapid, complete and kinetically invariant. Nat. Cell Biol. 2: 156–162
Gross A, McDonnell JM and Korsmeyer SJ (1999) BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 13: 1899–1911
Robertson JD, Enoksson M, Suomela M, Zhivotovsky B and Orrenius S . (2002) Caspase-2 acts upstream of mitochondria to promote cytochrome c release during etoposide-induced apoptosis. J. Biol. Chem. 13: 13
Guo Y, Srinivasula SM, Druilhe A, Fernandes-Alnemri T and Alnemri ES (2002) Caspase-2 induces apoptosis by releasing proapoptotic proteins from mitochondria. J. Biol. Chem. 277: 13430–13437
Lassus P, Opitz-Araya X, Lazebnik Y . Requirement for caspase-2 in stress-induced apoptosis before mitochondrial permeabilization. (2002) Science. 297: 1352–1354
Macho A, Hirsch T, Marzo I, Marchetti P, Dallaporta B, Susin SA, Zamzami N and Kroemer G (1997) Glutathione depletion is an early and calcium elevation is a late event of thymocyte apoptosis. J. Immunol. 158: 4612–4619
Curtin JF, Donovan M and Cotter TG (2002) Regulation and measurement of oxidative stress in apoptosis. J. Immunol. Methods 265: 49–72
Simonian PL, Grillot DA and Nunez G (1997) Bcl-2 and Bcl-XL can differentially block chemotherapy-induced cell death. Blood 90: 1208–1216
Acknowledgements
We thank Drs. SM Srinivasula, ES Alnemri (Thomas Jefferson University) for the caspase-9-DN, and G. Nunez (University of Michigan) for the pSFFV-FLAG.Bcl-2 constructs, and Ms. C Stanko (Cleveland Clinic Flow Cytometry Core) for help with flow cytometry. This work was supported by research grants from the National Institutes of Health to AA (CA81504 and CA82858) and GMC (HL29582).
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by G Salvesen
Rights and permissions
About this article
Cite this article
Chen, Q., Chai, YC., Mazumder, S. et al. The late increase in intracellular free radical oxygen species during apoptosis is associated with cytochrome c release, caspase activation, and mitochondrial dysfunction. Cell Death Differ 10, 323–334 (2003). https://doi.org/10.1038/sj.cdd.4401148
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.cdd.4401148
Keywords
This article is cited by
-
Genome-wide screening in the haploid system reveals Slc25a43 as a target gene of oxidative toxicity
Cell Death & Disease (2022)
-
Bioengineered gold nanoparticles using Cynodon dactylon extract and its cytotoxicity and antibacterial activities
Bioprocess and Biosystems Engineering (2021)
-
Doxorubicin Cytotoxicity in Differentiated H9c2 Cardiomyocytes: Evidence for Acute Mitochondrial Superoxide Generation
Cardiovascular Toxicology (2021)
-
Two novel anticancer compounds with minimum cardiotoxic property
BMC Pharmacology and Toxicology (2020)
-
Principles of fluoride toxicity and the cellular response: a review
Archives of Toxicology (2020)